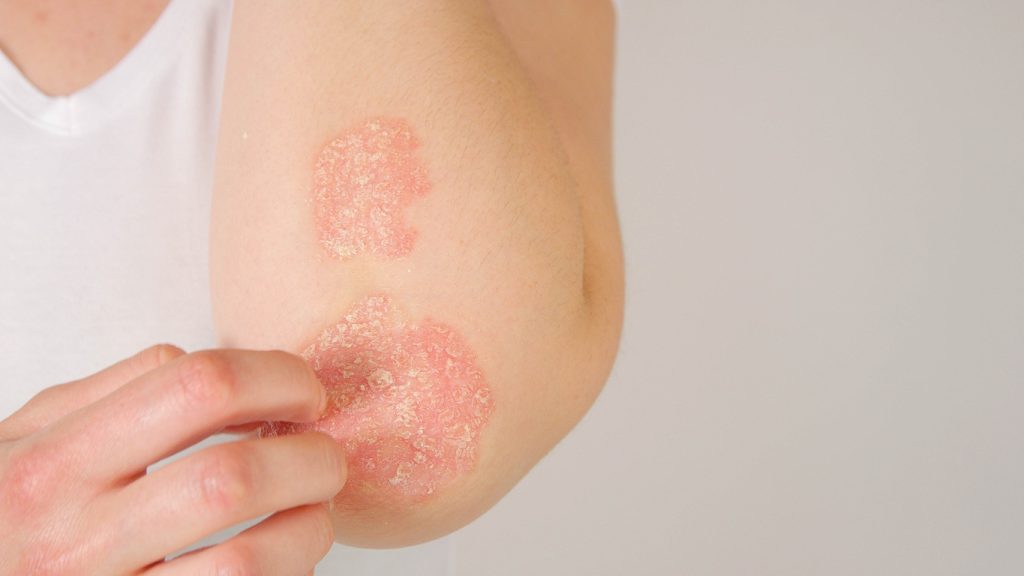
The U.S. Food and Drug Administration has cleared the Investigational New Drug (IND) application for VYN202, an oral small molecule BD2-selective BET inhibitor that may play a role in treating immuno-inflammatory disease, Vyne Therapeutics reports.
The company now plans to initiate a first-in-human Phase 1a single ascending dose/multiple ascending dose (SAD/MAD) trial in healthy volunteers this quarter and expects to report top line results from the SAD/MAD trial in the second half of this year.
“VYN202 is a highly selective and potent orally administered BET inhibitor that we believe has significant potential as a treatment option for autoimmune diseases. Clearance of the IND for VYN202 marks a major step forward in this effort,” says David Domzalski, President and CEO of Vyne, in a news release. “We look forward to initiating the Phase 1a trial in the coming weeks to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of VYN202.”
If the Phase 1a part of the trial is successfully completed, Vyne plans to initiate Phase 1b trials in subjects with moderate-to-severe plaque psoriasis and moderate-to-severe adult-onset rheumatoid arthritis, with top line results anticipated in the second half of 2025.
The VYN202 Phase 1a trial is a double-blind, placebo-controlled study in healthy volunteers and consists of SAD and MAD components. The study is expected to enroll approximately 64 healthy adult subjects into five SAD and three MAD cohorts to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of VYN202. If the Phase 1a part of the trial is successfully completed, Vyne plans to initiate Phase 1b trials in subjects with moderate-to-severe plaque psoriasis and moderate-to-severe adult-onset rheumatoid arthritis, with top line results anticipated in the second half of 2025.