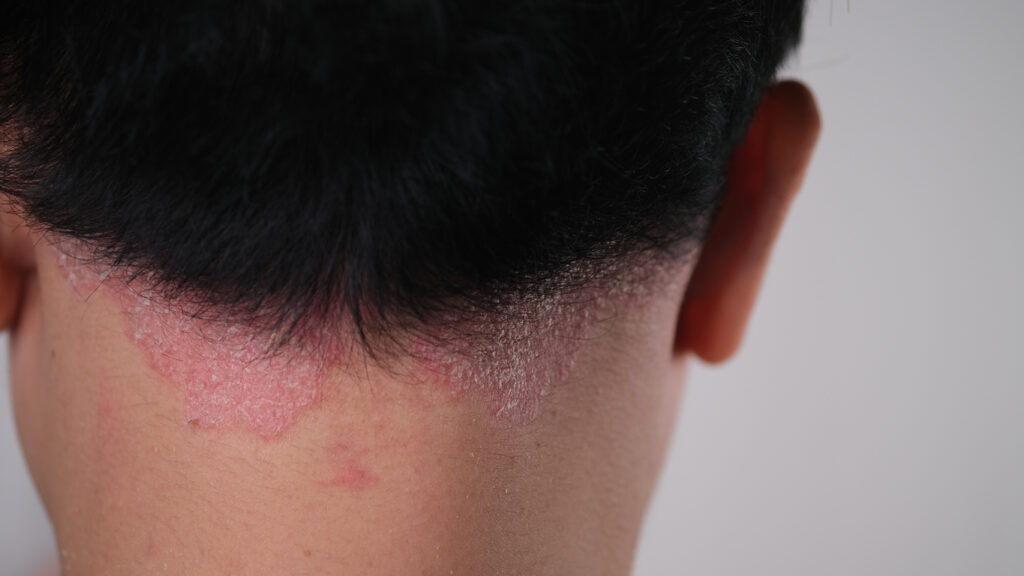
LEO Pharma’s Enstilar foam (LEO 90100) outperformed Daivobet ointment in a phase 3 trial of adults with stable plaque psoriasis in China, according to topline results.
Enstilar –a calcipotriol/betamethasone cutaneous foam –is built on the active ingredients of Daivobet. Both are Leo Products.
Once the full results of the new Phase 3 study are reported, LEO Pharma will proceed with preparations to submit Enstilar for approval in China. Enstilar foam has been U.S Food and Drug Administration-approved for the treatment of plaque psoriasis since 2015, and a generic version of the foam is available in the U.S.
The new trial compared the efficacy and safety of once daily application with Enstilar and Daivobet ointment for four weeks in 604 adult Chinese subjects with stable plaque psoriasis across 39 sites.
The primary objective of the study was to evaluate the efficacy of Enstilar compared with Daivobet ointment on severity and extent of stable plaque psoriasis. The secondary objective evaluated treatment safety. Exploratory objectives included evaluating health-related quality of life.
Enstilar showed superiority over Daivobet ointment in primary and secondary endpoints in the testing hierarchy, illustrating improved efficacy in reducing the severity and extent of stable plaque psoriasis. Overall, both Daivobet ointment and Enstilar were well tolerated. Safety profiles were consistent with previous trial findings with no new safety concerns identified.
“With these inspiring clinical results, we are very happy to be able to proceed towards submission of Enstilar to the Chinese authorities, but I am equally pleased that we will now proceed towards hopefully providing Chinese patients with a new treatment option for their psoriasis in addition to the existing portfolio offering from LEO Pharma China,” says Byron Yin, General Manager for LEO Pharma in China, in a news release.
Professor Zhang Jianzhong from Peking University People’s Hospital, the leading principal investigator of this study, adds:. “As a principal investigator in this study, I am delighted to see that Enstilar has achieved its primary and secondary endpoints in the Phase III clinical trial in Chinese patients, demonstrating superior efficacy to Daivobet with a good safety profile.”
Detailed results from the Enstilar China trial will be submitted for scientific presentation and publication at a later date.