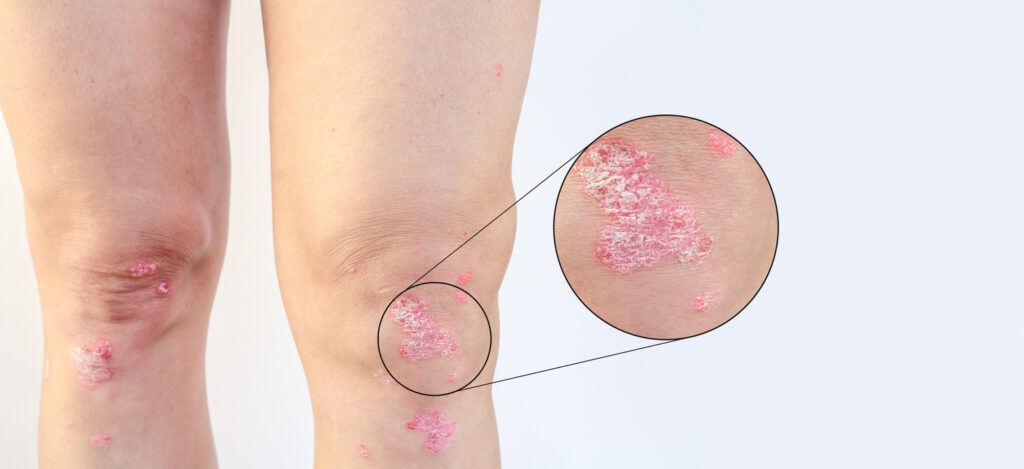
Johnson & Johnson submitted a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) seeking approval to include new evidence on the guselkumab (Tremfya) label for the inhibition of progression of structural damage in adults with active psoriatic arthritis (PsA).
The submission is supported by the Phase 3b APEX study in patients with active PsA, which achieved both its primary endpoint of reducing joint symptoms (ACR20) and its major secondary endpoint of inhibited progression of structural damage as measured by change in the modified van der Heijde-Sharp (vdH-S) score at 24 weeks, compared to placebo in bio-naïve patients. These data were presented at the European Alliance of Associations for Rheumatology (EULAR) 2025 Congress.
Additional data will be presented at future medical meetings.