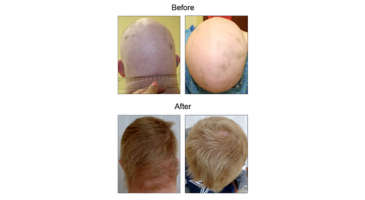
Janus kinase (JAK) inhibition may decrease the burden of autoimmune conditions in people with Down syndrome, according to a new study published in eLife.
Drawing upon their 2016 discovery that the interferon response is constantly activated in people with Down syndrome, researchers designed the trial to focus on the autoimmune and inflammatory skin conditions that are common in people with Down syndrome including alopecia areata, psoriasis, atopic dermatitis, and hidradenitis suppurativa. Study participants received tofacitinib (Xeljanz, Pfizer).
Researchers also monitored effects on co-occurring autoimmune conditions, such as autoimmune thyroid disease, celiac disease, and arthritis.
The study found improvements in skin pathology, with the most striking results seen for those affected by alopecia areata, as well as improvements in arthritis and decreased biomarkers of autoimmune thyroid disease.
Most study participants chose to remain on the medicine, often through off-label prescriptions, after completion of trial activities.
“Most importantly, we observed that major inflammatory markers elevated in Down syndrome that are known to cause autoimmunity were brought down to the normal range with this medicine, indicating that the immune system is being calmed down by this JAK inhibitor, while preserving strong immune function,” explains Joaquín Espinosa, PhD, Executive Director of the Linda Crnic Institute for Down Syndrome, Professor of Pharmacology at the University of Colorado Anschutz Medical Campus in Aurora CO, and one of the principal investigators in the clinical trial. “More data will be needed to define the safety profile of JAK inhibitors in Down syndrome, and we look forward to the completion of the trial and analysis of the full dataset.”
The study also reports a deep characterization of the immune system dysregulation characteristic of Down syndrome through analysis of clinical data and biospecimens collected by the ongoing Human Trisome Project study.
The Crnic Institute team analyzed clinical data and blood samples to characterize the pattern of autoimmune conditions and accompanying inflammatory processes in hundreds of research participants in the Human Trisome Project using multi-omics technologies. They observed that triplication of chromosome 21, or trisomy 21, leads to rapid onset of diverse autoimmune conditions during childhood, along with increased levels of many inflammatory factors and strong dysregulation of multiple immune cell types.
“One key observation is that elevation of multiple inflammatory markers and dysregulation of all branches of the immune system occurs from a very early age, even before any clinical manifestations of autoimmunity,” says Matthew Galbraith, PhD, Assistant Research Professor of Pharmacology at the University of Colorado Anschutz Medical Campus, and Director of the Data Sciences Program of the Crnic Institute and co-author of the study. “This points to a constitutive state of immune dysregulation triggered by the extra chromosome that eventually leads to the appearance of multiple autoimmune conditions, with variations in timing and severity among individuals.”
“Since 2016 we have been hypothesizing that the class of medicines known as JAK inhibitors will provide therapeutic benefits in this population,” explains Angela Rachubinski, PhD, Assistant Research Professor of Pediatrics, Director of the Clinical and Translational Sciences Program at the Crnic Institute, and lead author of the paper. “Although JAK inhibitors have been approved for a range of autoimmune and inflammatory conditions in the general population, this clinical trial, which started activities back in 2020, provides the first systematic investigation of the effects of a JAK inhibitor in people with Down syndrome.”
The Crnic Institute study team is now overseeing a second trial testing the safety and efficacy of the JAK inhibitor relative to other medicines for treating the condition known as Down Syndrome Regression Disorder, and a third trial focused on children with Down syndrome is expected to start recruitment in late 2024.
“We are very grateful to the scientists and physicians at the Crnic Institute for their transformative research that is already translating into improved medical care and health outcomes for the amazing people with Down syndrome who we serve,” says Michelle Sie Whitten, President & CEO of the Global Down Syndrome Foundation. “We are proud that GLOBAL’s advocacy work with Congress and with the National Institutes of Health (NIH) has led to the establishment of the trans-NIH Down syndrome funding project, INCLUDE, that underwrites this and numerous other groundbreaking studies and clinical trials.”
PHOTO CREDIT: The Linda Crnic Institute for Down Syndrome