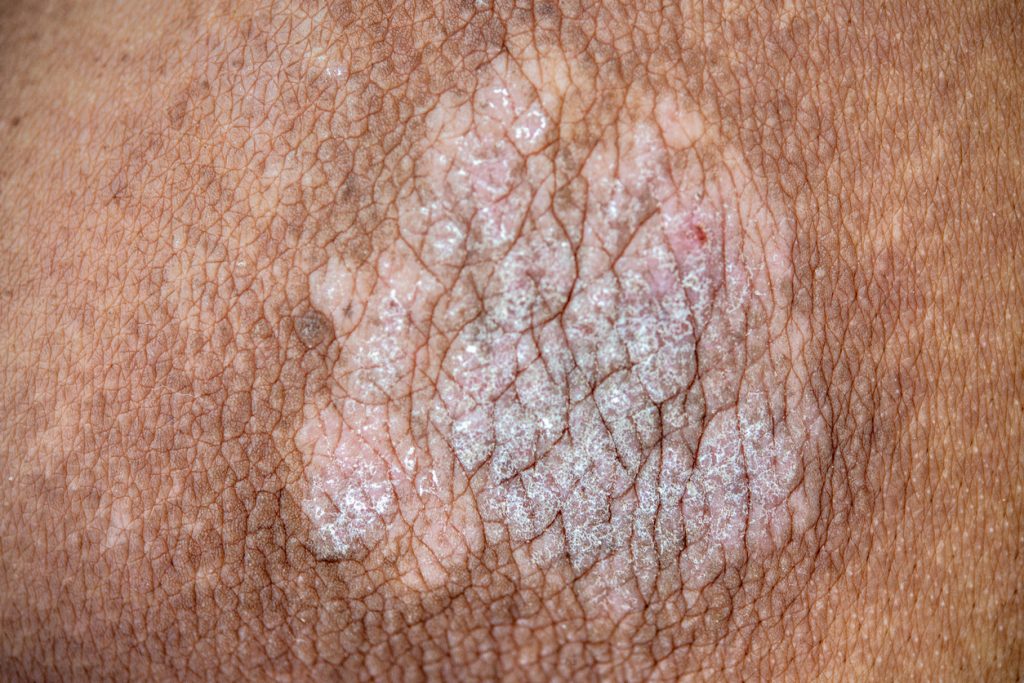
InnoCare Pharma’s novel TYK2 (Tyrosine Kinase 2) inhibitor ICP-488 met its primary endpoint in adults with moderate-to-severe plaque psoriasis in a phase II clinical study.
ICP-488 is an oral TYK2 allosteric inhibitor. By binding to the JH2 domain, ICP-488 blocks the signal transduction of interleukin (IL)-23, IL-12, type 1 interferon (IFN) and other inflammatory cytokine, thereby inhibiting the pathological process of autoimmune and inflammatory diseases.
Among psoriasis patients treated for 12 weeks, ICP-488 achieved multiple efficacy endpoints including Psoriasis Area and Severity Index (PASI) 75, PASI 90, PASI 100 (improvement of at least 75%, 90% and 100% in PASI score from baseline) and static Physician’s Global Assessment (sPGA) 0/1 (score of 0 ‘clear’ or 1 ‘almost clear’) in both the 6mg and 8mg dosing group respectively.
Following the 12-week treatment with 6mg or 9mg once-daily dosing of ICP-488, PASI 75 reached 77.3% and 78.6% respectively, compared to 11.6% for patients receiving placebo; PASI 90 reached 36.4% and 50.0% respectively, compared to 0% for patients receiving placebo; PASI 100 reached 11.4% and 11.9% respectively, compared to 0% for patients receiving placebo; sPGA 0/1 reached 70.5% and 71.4% respectively, compared to 9.3% for patients receiving placebo.
ICP-488 demonstrated good tolerability and a favorable safety profile, with most treatment emergent adverse events (TEREs) and treatment-related adverse events (TRAEs) being mild or moderate.
“Psoriasis requires long-term management, and there remains a significant unmet medical need for new treatments,” says Dr. Jasmine Cui, Co-founder, Chairwoman and CEO of InnoCare, in a news release. “We are excited to see the positive results from the phase II study of ICP-488, and we will further accelerate its clinical development to benefit patients with psoriasis and other autoimmune diseases.”
The multicenter, randomized, double-blind, placebo-controlled phase II clinical study aimed at evaluating the efficacy, safety, pharmacokinetic ans pharmacodynamic characteristics of ICP-488 in Chinese adult patients with moderate to severe plaque psoriasis. A total of 129 patients were enrolled in this study. Patients were randomly assigned to one of three treatment groups in a 1:1:1 ratio, a 6mg once-daily group, a 9mg once-daily group, and a placebo group, for 12 consecutive weeks of treatment.