INmune Bio, Inc. is on track to submit a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) and Marketing Authorization Application (MAA) in the UK and EU for CORDStrom in Recessive Dystrophic Epidermolysis Bullosa (RDEB).
CORDStrom is a patent-pending, off-the-shelf, advanced mesenchymal stromal cell (MSC) platform developed to treat complex inflammatory diseases.
The submission will be supported by data from the MissionEB clinical trial investigating CORDStrom as a disease-modifying therapy for treating RDEB in pediatric patients. This trial showed a favorable benefit-risk profile in support of the intended applications for marketing authorization.
The Company participated in a Type C meeting with the FDA, the outcome of which provided information related to chemistry, manufacturing, and controls, and other regulatory topics in anticipation of the Company’s efforts to prepare and submit a BLA.
The FDA granted CORDStrom a rare pediatric disease designation (RPDD) for treating EB on December 13, 2024, ahead of the priority review voucher (PRV) sunset period. As such, CORDStrom remains eligible to receive a PRV if approved by the FDA on or prior to September 30, 2026, which date may be extended by Congress. If granted, a PRV can be redeemed to receive a priority review for a different product, or it may be transferred or sold.
Additionally, the FDA granted CORDStrom an orphan drug designation (ODD) on January 6, 2025.
The Company intends to prepare for a pre-BLA meeting to discuss particulars of its planned BLA submission, with the intent to submit a BLA in 2025 seeking approval of CORDStrom for the treatment of RDEB in pediatric patients. Concurrently, the Company will also prepare to submit MAAs to the EU and UK in 2026.
About MissionEB
The MissionEB study, led by Dr. Anna Martinez and team at the Great Ormond Street Hospital (GOSH) London, U.K., in collaboration with clinicians from Birmingham’s Children’s Hospital, was a double-blind, placebo-controlled, cross-over study evaluating the safety and efficacy of CORDStrom in 30 pediatric patients (age <16 years) in the UK with intermediate or severe RDEB. Subjects were randomized to CORDStrom or placebo and received two intravenous infusions two weeks apart. Half of the patients were treated with CORDStrom and then crossed over to placebo following a washout period, and the other half were treated with a placebo and then crossed over to CORDStrom. Efficacy was assessed at 3- and 6-months from the first infusion per study arm. All patients are included in the 3- and 6-month efficacy assessment of both placebo and CORDStrom.
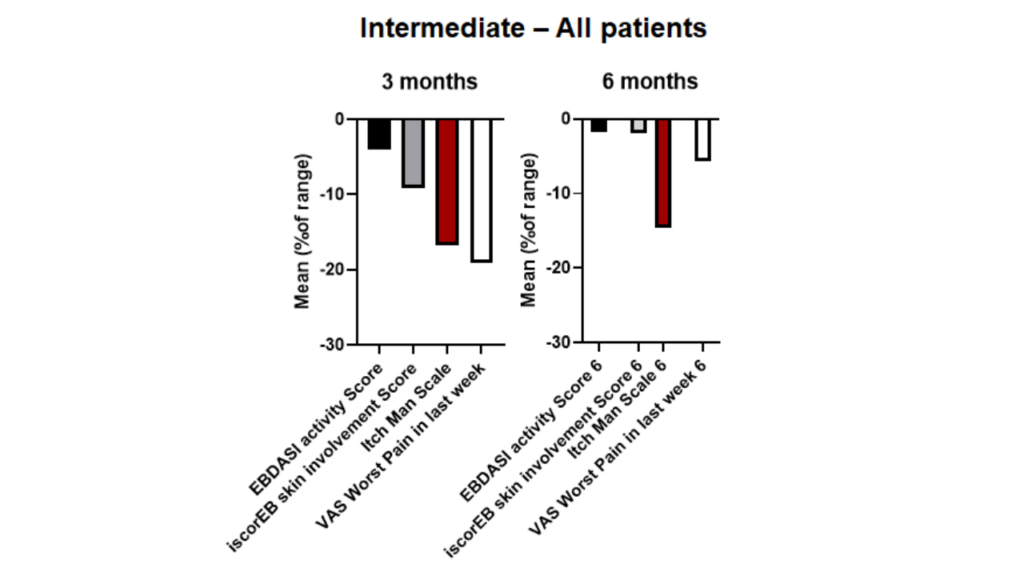
CORDStrom was extremely well tolerated, with no serious adverse events related to CORDStrom reported at 3 months or 6 months post-treatment across all age and RDEB severity patient sub-types. In children with severe disease, CORDStrom reduced itch at 3 months, leading to a sustained reduction of over 27% at 6 months. These results demonstrate that a clinically meaningful decrease in itch severity is sustained over time. In children with intermediate disease severity, CORDStrom provided a broader range of improvements, including reduced skin involvement, less pain, and a large reduction in itch. In younger children with RDEB (age < 10 years), CORDStrom improved skin scores, indicating better skin integrity and reduced disease activity.
Interviews with subjects and caregivers strongly support the clinical benefits of CORDStrom, as both caregivers and patients were able to correctly identify which treatment had been CORDStrom and which had been placebo in this cross-over study.
With great interest from the patients to continue therapy, the Company intends to support a 12-month open-label study at GOSH, including all patients enrolled in the MissionEB study, where patients will receive three cycles of CORDStrom therapy at time 0, 4, and 8 months.