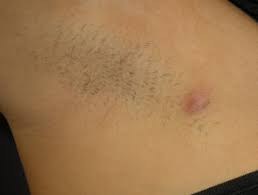
Twice-daily application of ruxolitinib (Opzelura, Incyte) cream, 1.5% may help treat milder hidradenitis suppurativa (HS), according to new phase 2 data presented at the annual meeting of the American Academy of Dermatology in San Diego, CA.
Specifically, patients with Hurley stage 1 or 2 HS who applied ruxolitinib cream achieved a significantly greater reduction in abscess and inflammatory nodule count (AN count) from baseline at week 16 when compared to those who applied the vehicle control.
“The Phase 2 study evaluating ruxolitinib cream, 1.5% in patients with HS met its primary endpoint, reinforcing the efficacy and safety profile of ruxolitinib cream 1.5%, demonstrating potential as a novel treatment option for people living with HS,” study author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center in Boston, tells TDD. Exactly how ruxolitinib cream helps treat HS is not fully understood yet. “Over-activity of the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway is believed to drive inflammation involved in the pathogenesis and progression of HS, “ explains Dr. Porter, adding that oral JAK inhibitors are also a promising therapy for patients with HS.
Select Secondary Endpoints Met
In the study, more than three quarters of on-treatment patients achieved at least a 50% reduction in AN count (AN50), 54.2% achieved a 75% reduction (AN75), 20.8% achieved 90% reduction (AN90), and 20.8% achieved complete clearance (100% reduction, AN100), surpassing the 56.3%, 25.0%, 12.5% and 12.5% reductions, respectively, in the vehicle control group, the study found.
The majority (79.2%) of patients in the ruxolitinib cream 1.5% group met the criteria for Hidradenitis Suppurativa Clinical Response (HiSCR), which indicates a 50% or greater reduction in AN count without an increase in abscesses or draining fistulas, compared to 50% of patients in the vehicle control group. Moreover, HS patients treated with ruxolitinib cream 1.5% showed a greater mean reduction in the International Hidradenitis Suppurativa Severity Score System (IHS4) score compared to baseline at Week 16 compared to the vehicle group.
HS patients treated with ruxolitinib cream 1.5% showed a change -1.85 and -1.42 from baseline in the Skin Pain Numeric Rating Scale (NRS) and Itch NRS at week 16, respectively, versus a -2.61 and -2.75 change from those in the vehicle control group. Due to patient eligibility criteria, patients studied did not have high itch or skin pain scores at baseline. Additional research is needed to evaluate treatment impact on skin pain and itch scores.
The study results showed that ruxolitinib cream 1.5% was generally well-tolerated. Treatment-emergent adverse events (TEAEs) occurred in 38.2% of patients who applied ruxolitinib cream 1.5% versus 42.9% of patients who applied vehicle control. The most common TEAEs among patients receiving ruxolitinib cream 1.5% were COVID-19 and nasopharyngitis. Discontinuation due to TEAEs were infrequent, and no serious TEAEs were reported in the ruxolitinib cream 1.5% group.
Unmet Need in HS: Where Might Ruxolitinib Cream Fit In?
“The prevalence of HS in the population is thought to be about 0.5-1%, and we have made a lot of progress investigating therapies for moderate-to-severe HS, but there are currently no approved therapies for milder HS and the current standard of care is often inadequate for these patients,” she tells TDD.
“Given the debilitating nature of HS, it can have a profoundly negative effect on patients’ quality of life, making it imperative that effective treatment options are developed that can better control the disease and manage signs and symptoms.”
Ruxolitinib cream could be used both as monotherapy or potentially in combination with other therapies for HS in the future, she says. “We currently often have to employ multiple treatments together to treat patients effectively.”
PHOTO CREDIT: DermNetNZ