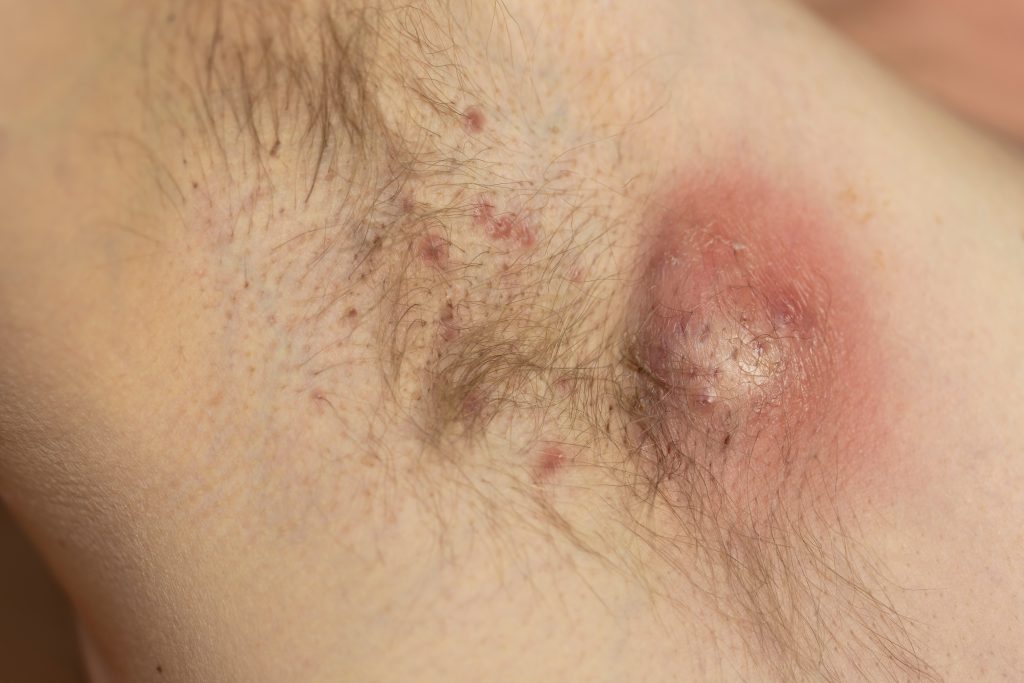
Bimekizumab (Bimzelx, UCB) continues to provide symptom relief in hidradenitis suppurativa (HS) for up to three years, according to new research presented at the European Academy of Dermatology and Venereology (EADV) 2025 Congress in Paris, France.
Improvements in the stringent endpoint Hidradenitis Suppurativa Clinical Response (HiSCR) 100, a 100% reduction in the total abscess and inflammatory nodule count from baseline, at one year, were sustained and further improved in 50.1% of patients after three years.
What’s more, improvements in quality of life as measured by Dermatology Life Quality Index (DLQI) 0/1 at year one were sustained by 38.1% of patients.
Of patients who achieved an International Hidradenitis Suppurativa Severity Score (IHS4)-100, a European clinical metric to assess the severity of HS at Year One, 64.3% achieved and maintained this complete resolution of inflammatory lesions through two years.
Across the IHS4 thresholds, patients treated earlier had improved outcomes at two years, the researchers report.