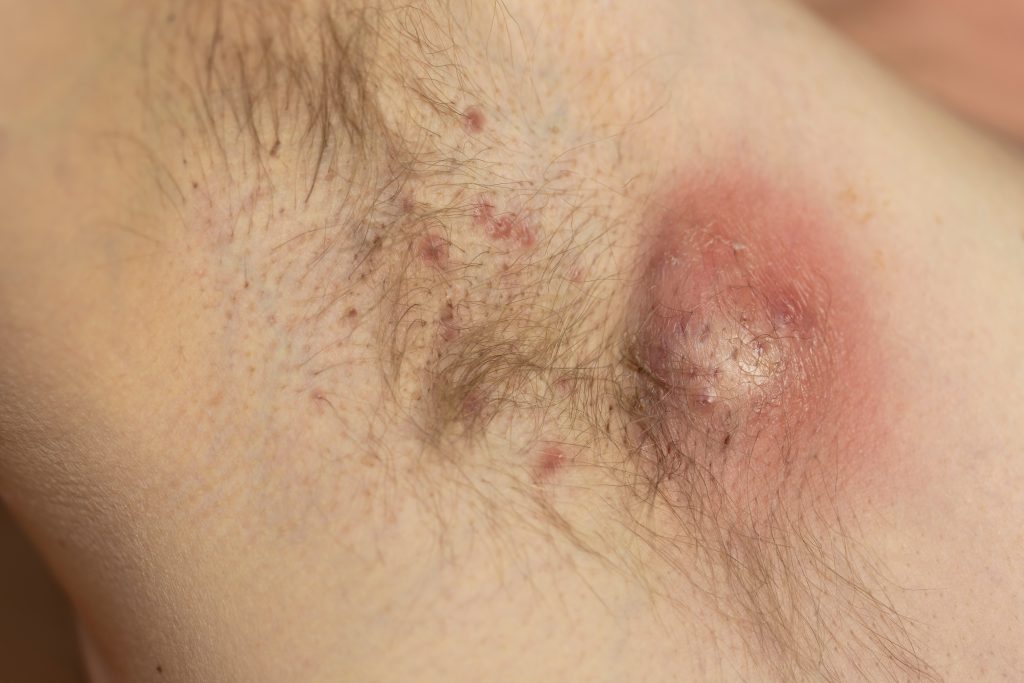
Zura Bio Limited is launching TibuSHIELD, a global Phase 2 clinical study evaluating tibulizumab in adults with moderate-to-severe hidradenitis suppurativa (HS).
Tibulizumab inhibits B-cell activating factor (BAFF) and interleukin-17A (IL-17A) pathways.
TibuSHIELD is designed to enroll approximately 180 adults with moderate-to-severe HS across the United States, Canada, and Europe. The study will evaluate tibulizumab over a 28-week period, comprising a 16-week primary efficacy assessment and a 12-week safety follow-up, with an optional OLE.
The primary endpoint of the study is the percent change from baseline in total abscess and nodule (AN) count at Week 16. Secondary endpoints include the proportion of participants achieving Hidradenitis Suppurativa Clinical Response 50 (HiSCR50) or HiSCR75, defined as at least a 50% or 75% reduction in abscess and inflammatory nodule count (AN) count without an increase in abscesses or draining fistulas at Week 16. Key safety assessments include the assessment of tolerability, and monitoring for adverse events.
Topline results for the primary efficacy endpoint at Week 16 are expected in the third quarter of 2026.
“Hidradenitis suppurativa remains a difficult condition to manage, with many patients experiencing persistent disease activity despite currently available treatments,” says Alexa B. Kimball, MD, MPH, Professor of Dermatology at Harvard Medical School, and President and CEO of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center in Boston, MA. “Studying tibulizumab represents an important opportunity to evaluate an investigational therapy designed to target multiple aspects of the inflammatory process in HS. We look forward to investigating this approach for individuals living with this challenging disease.”
More on Tibulizumab
Tibulizumab is an investigational, humanized, tetravalent dual-antagonist antibody engineered by fusing Ixekizumab (Taltz, Eli Lilly and Company) and tabalumab to bind to and neutralize both IL-17A and BAFF. It is currently being evaluated in two Phase 2 clinical studies in adults with systemic sclerosis and hidradenitis suppurativa. Prior to in-licensing, Phase 1/1b studies were conducted in patients with Sjögren’s syndrome and rheumatoid arthritis.
Tibulizumab is an investigational compound and has not been approved for marketing by the U.S. Food and Drug Administration (FDA) or any other regulatory authority.