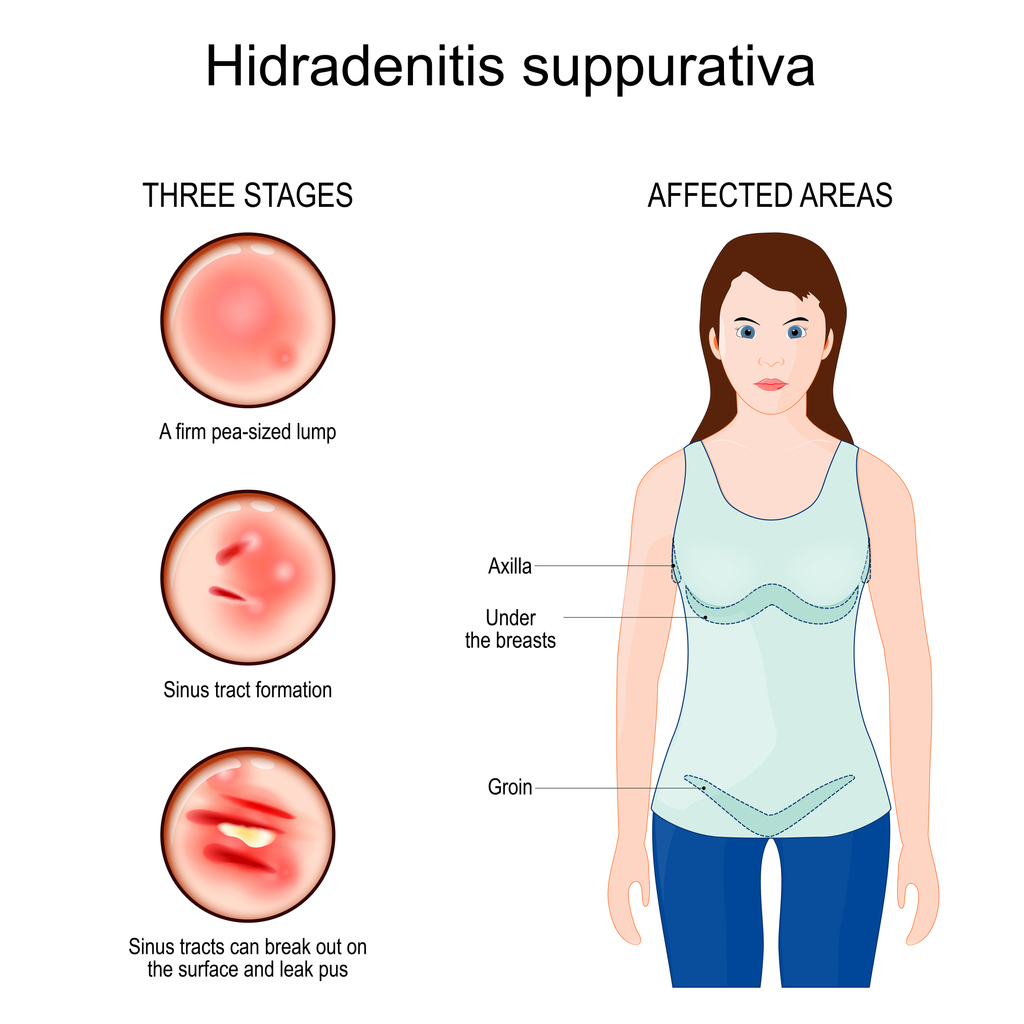
The first patients have been screened at a U.S. trial site in MoonLake Immunotherapeutics’ global Phase 3 clinical program, VELA, evaluating sonelokimab in hidradenitis suppurativa (HS).
Sonelokimab is a Nanobody designed to directly target sites of inflammation by inhibiting the IL-17A/A, IL-17A/F, and IL-17F/F dimers and to penetrate difficult-to-reach inflamed tissues.
The initiation of this Phase 3 program follows the announcement in February 2024 of the successful outcome of MoonLake’s end-of-Phase 2 interactions with the U.S. Food and Drug Administration (FDA), as well as positive feedback from its interactions with the European Medicines Agency (EMA), with both regulatory bodies unanimously supporting MoonLake’s proposed approach for advancing its Phase 3 program in HS. Sonelokimab is being assessed in two lead indications, HS and psoriatic arthritis (PSA), and the Company is pursuing other indications in dermatology and rheumatology.
Sonelokimab is not yet approved for use in any indication.
The Phase 3 VELA program is expected to enroll 800 patients across VELA-1 and VELA-2. Both trials are identical in design comparing a single 120mg dose of sonelokimab to placebo with the higher measure of clinical response, Hidradenitis Suppurativa Clinical Response (HiSCR) 75, as the primary endpoint reading out at week 16. From week 16, all patients will receive the 120mg dose of sonelokimab through to 52 weeks, followed by an open-label extension for up to two years. The Phase 3 program will use a protocol design consistent with the Phase 2 MIRA trial, which identified the optimal dose of sonelokimab for HS. The topline primary endpoint readout (week 16) from the VELA program is expected as of mid-2025.
“With real-world data indicating that at least two million Americans have been diagnosed with and treated for HS, the launch of our Phase 3 VELA program with our Nanobody sonelokimab, using the higher clinical measure of HiSCR75 as the primary endpoint and a straightforward, proven study design is a landmark moment in our efforts to develop novel treatment options for patients suffering with this under-diagnosed and under-treated condition,” says Kristian Reich, Founder and Chief Scientific Officer at MoonLake, in a news release. “ We are making significant progress in establishing clinical trial sites to enroll 800 patients, and we eagerly anticipate reporting the week 16 primary endpoint readout around mid-2025.”
Hadar Lev-Tov, MD, MAS, Associate Professor, Director Wound Healing Fellowship, President Hidradenitis Suppurativa Foundation, Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery, University of Miami, Miller School of Medicine, adds: “With only two FDA approved biologics, there is still an urgent need for new treatment options that treat all patient types and lesions, with the opportunity for inflammatory remission. The unique characteristics and mode of action of MoonLake’s Nanobody, sonelokimab to effectively inhibit IL-17F in addition to IL-17A in deep tissue inflammation has to date shown promising outcomes, highlighting the importance of this Phase 3 program, and placing HS at the forefront of dermatological innovation.”
Seth B Forman, MD, Principal Investigator at CenExel-FCR, agrees. “It is with great enthusiasm that I am participating as an investigator in the Phase 3 VELA program investigating the Nanobody sonelokimab for HS, signifying a substantial advancement in addressing the critical unmet need for more treatment options for individuals living with HS. As a physician, I witness first-hand the immense demand for novel treatment options for people living with HS, particularly those that can achieve elevated response thresholds (e.g., HiSCR75 and beyond). The findings from the Phase 2 MIRA trial offer valuable insights into what may be possible as we work with our patients to establish more ambitious treatment goals and alleviate the disease burden of this debilitating condition.”
Sonelokimab in PsA
For PsA, Phase 3 initiation is anticipated in Q4 2024 following the announcement in March 2024 of the full dataset from the global Phase 2 ARGO trial (M1095-PSA-201) evaluating the efficacy and safety of the Nanobody sonelokimab over 24 weeks in patients with active PsA. Significant improvements were observed across all key outcomes, including approximately 60% of patients treated with sonelokimab achieving an American College of Rheumatology (ACR) 50 response and Minimal Disease Activity (MDA) at week 24. This followed the positive top-line results in November 2023, where the trial met its primary endpoint with a statistically significant greater proportion of patients treated with either sonelokimab 60mg or 120mg (with induction) achieving ACR50 response compared to those on placebo at week 12. All key secondary endpoints in the trial were met for the 60mg and 120mg doses with induction. The safety profile of sonelokimab in the ARGO trial was consistent with previous trials with no new safety signals detected.