Benzoyl Peroxide-based acne medications can become unstable and form benzene, a known human carcinogen, according to new research from Valisure, an independent laboratory focused on testing the purity and safety of pharmaceutical drugs and consumer products.
Valisure is now requesting an investigation and market withdrawal of BPO-containing products via a new petition to the U.S. Food and Drug Administration (FDA).
Results show that on-market Benzoyl Peroxide (BPO) products can form more than 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene, and the current evidence suggests that this problem applies broadly to BPO products currently on the market.
High levels of benzene were not only detected inside BPO products but also in the air around incubated BPO products, showing that benzene can leak out of some product packages and pose a potential inhalation risk. Incubation of a Proactiv product at the temperature of a hot car (70°C) resulted in the detection of benzene in a compact car’s volume of air at ~1,270 times the Environmental Protection Agency’s (“EPA”) calculated threshold for increased cancer risk by long-term inhalation exposure to benzene.
Valisure’s tests on dozens of prescription and over-the-counter BPO products suggest that currently formulated BPO medications are fundamentally unstable and can generate unacceptably high levels of benzene when handled or stored at higher temperatures that the products may be exposed to during handling by consumers. Benzene can be produced in the product itself and potentially escape into the surrounding air.
“There is not a safe level of benzene that can exist in any skin care product, over the counter or prescription,” says TDD Editorial Advisory Board Member Christopher Bunick, MD, PhD, Associate Professor of Dermatology at Yale University in New Haven, CT, in a news release. “The current data on BPO degrading into high levels of benzene is extremely concerning given its prominent use in skin care, and this study should serve as another wake-up call for improved manufacturing and quality control of consumer healthcare products.”
Only BPO-containing acne treatment products have this issue of forming high levels of benzene. Other acne treatment products tested by Valisure such as those containing salicylic acid or adapalene do not appear to have this problem.
“This discovery of benzoyl peroxide’s fundamental instability and formation of benzene is substantially different than Valisure’s previous findings of benzene in sunscreens, hand sanitizers, and other consumer products,” says David Light, Valisure’s Co-Founder and President. “The benzene we found in sunscreens and other consumer products were impurities that came from contaminated ingredients; however, the benzene in benzoyl peroxide products is coming from the benzoyl peroxide itself, sometimes at hundreds of times the conditional FDA limit. This means the problem broadly affects benzoyl peroxide products, both prescription and over-the-counter, and necessitates urgent action.”
Drug Instability & Valisure Data:
“Figure 1” from Valisure’s Petition; the mechanism of BPO degrading into benzene:
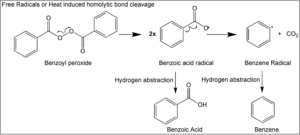
There is a precedent for broad, global action on drug instability with the medication ranitidine. In response to Valisure’s 2019 Citizen Petition on the inherent instability of ranitidine (Zantac,
GlaxoSmithKline PLC) and its formation of the probable human carcinogen N-nitrosodimethylamine (“NDMA”), FDA requested that manufacturers withdraw all ranitidine drug products from the market due to the FDA’s finding that “NDMA has been found to increase significantly in samples stored at higher temperatures, including temperatures the product may be exposed to during distribution and handling by consumers.” Published data shows that Zantac was stability tested at 70°C for two weeks and formed approximately 1 times the regulatory limit for NDMA; whereas Valisure’s testing indicates that BPO products can form over 800 times the conditional regulatory limit for benzene in two weeks at the lower temperature of 50°C.
Valisure now presents substantial evidence that BPO drug products are unstable and, at such temperatures of distribution and consumer handling, can form hundreds of times any FDA regulatory guidance for acceptable benzene levels. Valisure’s tests found benzene at high levels when BPO products were incubated at 37°C (98.6°F – body temperature), 50°C (122°F – accepted pharmaceutical stability testing temperature) and 70°C (158°F – hot car temperature). Valisure performed tests using both traditional gas chromatography-mass spectrometry (“GC-MS”) and a technology platform uniquely suited for the analysis of benzene from the air called selected-ion flow-tube mass spectrometry (“SIFT-MS”).
Valisure performed initial GC-MS analysis on 175 acne treatment products, 99 containing BPO and 76 containing other ingredients, most commonly salicylic acid or adapalene. All 76 non-BPO products had no detectable benzene or values below 2 ppm, and 94 of 99 BPO products contained benzene without any elevated temperature incubation. An initial stability study of 5 products using 37°C, 50°C and 70°C revealed that dozens of ppm of benzene can form in just a few weeks at 37°C, hundreds of ppm at 50°C, and 70°C the apparent degradation of BPO would often lead product packaging to burst. Therefore, 50°C was chosen as a stability temperature for a broader study of 66 BPO-containing products. In 18 days of stability testing at 50°C, Valisure detected over 1,500 ppm of benzene produced in 2 products, over 100 ppm in 17 products, and over 10 ppm in 42 products.
Valisure’s SIFT-MS analysis confirmed that substantial amounts of benzene can form in a BPO product and leak outside of the container into the surrounding air. In less than 1 day of incubation at 70 °C, the amount of benzene produced in the air was approximately equivalent to 29 ppm of benzene in the BPO product. That same amount of benzene detected in the air from incubation at 70°C (the temperature of a hot car) of one Proactiv product, if dispersed in a 100 cubic foot compact vehicle, would result in benzene levels in the car at approximately 1,270 times the EPA calculated threshold (further described below) for increased cancer risk by long-term inhalation exposure to benzene.
BPO Instability Well Known in Other Industries
BPO has been used in the polymer industry and its decomposition into benzene has been studied, with concern raised regarding the carcinogenic implications. In 1994, a paper was published by researchers at Denmark’s Department of Environmental Chemistry titled “Formation of benzene by hardeners containing benzoyl peroxide and phthalates” and stated: “Recently, during the investigation of benzene residues in chemical products (Rastogi 1993a), it was observed that the benzene content in benzoyl peroxide-containing hardeners of two-component repair-sets (fillers, elastomers) were >2 % (w/w) [20,000 ppm]. Benzene is carcinogenic (IARC1982), and its use in consumer and industrial products is generally avoided.”
Some companies in the chemical industry have actively attempted to address the BPO instability problem as it relates to plastics. At least one patent application was filed by the chemical company Akzo Nobel N.V. in 1997 for “reducing the rate of free benzene and/or benzene derivative formation in BPO formulations based on organic plasticizers.”
Valisure investigated a few common antioxidants in a model system of therapeutically relevant 10% BPO in glycerol. The results show that simply the addition of an antioxidant can substantially reduce the formation of benzene by over 98%. These and other techniques that Valisure has investigated could help address the BPO degradation problem and fix it for future drug formulations.
“The potential harm posed by benzene exposure cannot be ignored, and only underscores the critical need for independent quality assurance pioneered by Valisure,” says Chip Phillips, Chief Executive Officer of Valisure. “At Valisure, we’re constantly expanding not only our technical capabilities but also the avenues in which we can work with the industry to address and recognize quality for the benefit of all stakeholders in healthcare.”
Benzene toxicity in humans has been well-established for over 120 years. A study from 1939 on benzene stated that “exposure over a long period to any concentration of benzene greater than zero is not safe,” which is a comment reiterated in a 2010 review of benzene research specifically stating, “There is probably no safe level of exposure to benzene, and all exposures constitute some risk in a linear if not supra linear, and additive fashion.” Many epidemiological studies of petroleum workers exposed to benzene by inhalation have associated the chemical with the development of cancers of blood tissues, such as leukemia, at continued exposure to levels as low as 1 ppm.
FDA recognizes the serious danger of benzene and lists it as a “Class 1 solvent” that “should not be employed in the manufacture of drug substances, excipients, and drug products because of their unacceptable toxicity. However, if their use is unavoidable to produce a drug product with a significant therapeutic advance, then their levels should be restricted,” and benzene is restricted to 2 ppm for these particular circumstances.
In December 2023, the FDA took significant action through new guidance to tackle benzene contamination that was first identified by Valisure’s March 2021 FDA Citizen Petition detecting benzene in hand sanitizers, followed by a variety of consumer products including, sunscreens, antiperspirants, and dry shampoos. In the new guidance, the FDA urged manufacturers to reformulate various drug products containing “carbomer” gelling agents, which, as Valisure’s Hand Sanitizer Petition pointed out, pose a high risk of benzene contamination.
The EPA strictly regulates benzene in drinking water and monitors it in the air because, as stated by the EPA and numerous global regulatory agencies, “benzene can cause leukemia.” The long-established epidemiological data in humans is utilized by EPA to determine that a lifetime exposure of 0.4 ppb, or 0.0004 ppm, of benzene in air can lead to one additional cancer case in 100,000 exposed persons.
Calls for Action on Drug Quality Issues:
European regulators have recognized the importance of independent testing and established a multi-state system of over 70 “Official Medicines Control Laboratories (“OMCLs”) which are discussed in a European Medicines Directorate document: “By testing medicines independently of manufacturers (that is, without conflicts of interest and with guaranteed impartiality), the OMCLs have a fundamental role in ensuring the quality, safety and efficacy of medicines. When it comes to medicines in Europe, OMCLs play a major role in contributing to the overall welfare of patients and animals.”
The U.S. Department of Defense has recently engaged with Valisure to more closely study drug products used by the Military Health System and help develop systems to meaningfully incorporate independent testing into drug purchasing. From the recent announcement of this engagement: “By creating much-needed transparency in drug quality, this study will enable conscientious manufacturers to be able to better compete and allow major purchasers of drugs, like the Department of Defense and Veterans Administration, to reward good manufacturers and exclude substandard medicines from being consumed by the military and veterans, and serve as a model for broader adoption throughout the United States to benefit all American patients.”
Bi-partisan support in Washington has been growing for taking action on improving drug quality, addressing drug shortages, and specifically for independent chemical testing of medications.
“For years, I have fought to advance legislation – the Recall Unsafe Drugs Act – giving the FDA mandatory recall authority over drugs so the Agency can fully use its regulatory authority to protect public health,” says Congresswoman Rosa DeLauro (D-CT). “The discovery made by Valisure regarding Benzoyl Peroxide acne treatment products is deeply troubling and gives renewed importance to the need to empower FDA to immediately act once we are made aware of the dangers of prescription or over-the-counter drugs. Benzoyl Peroxide products saturate the current market, and millions of consumers are unknowingly using a product that increases their exposure to life-threatening carcinogens. We must immediately act to protect consumers and ensure that FDA has the power to do so.”
“Valisure’s findings emphasize the overwhelming need for robust independent quality assurance testing for pharmaceuticals,” adds Congressman Neal Dunn, MD (R-FL). “As a doctor and a Congressman, ensuring the safety of all Americans is of the utmost importance. With more independent research, we can quickly act to remove harmful products from the market when found to be unsafe.”


