Hidradenitis Suppurativa
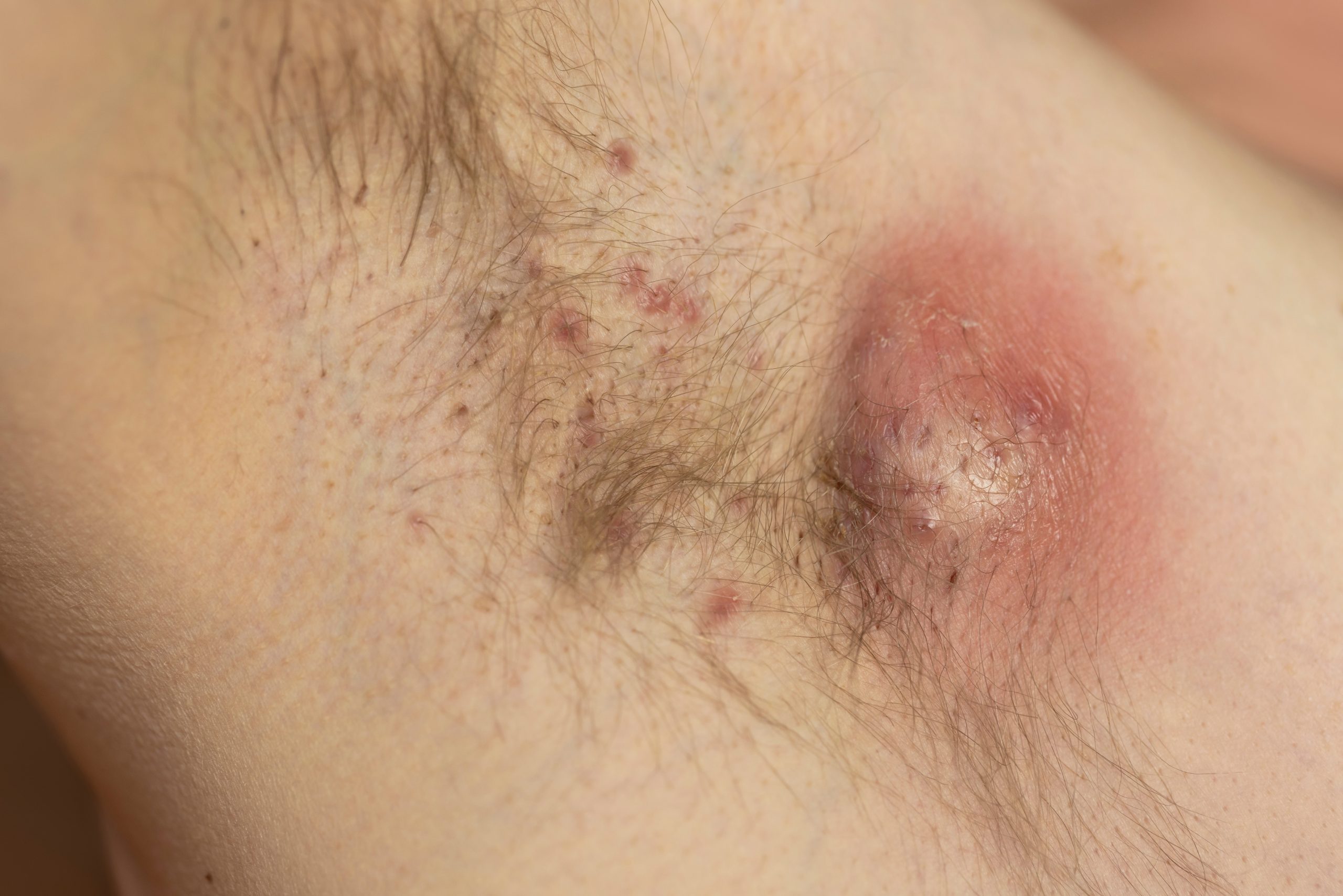






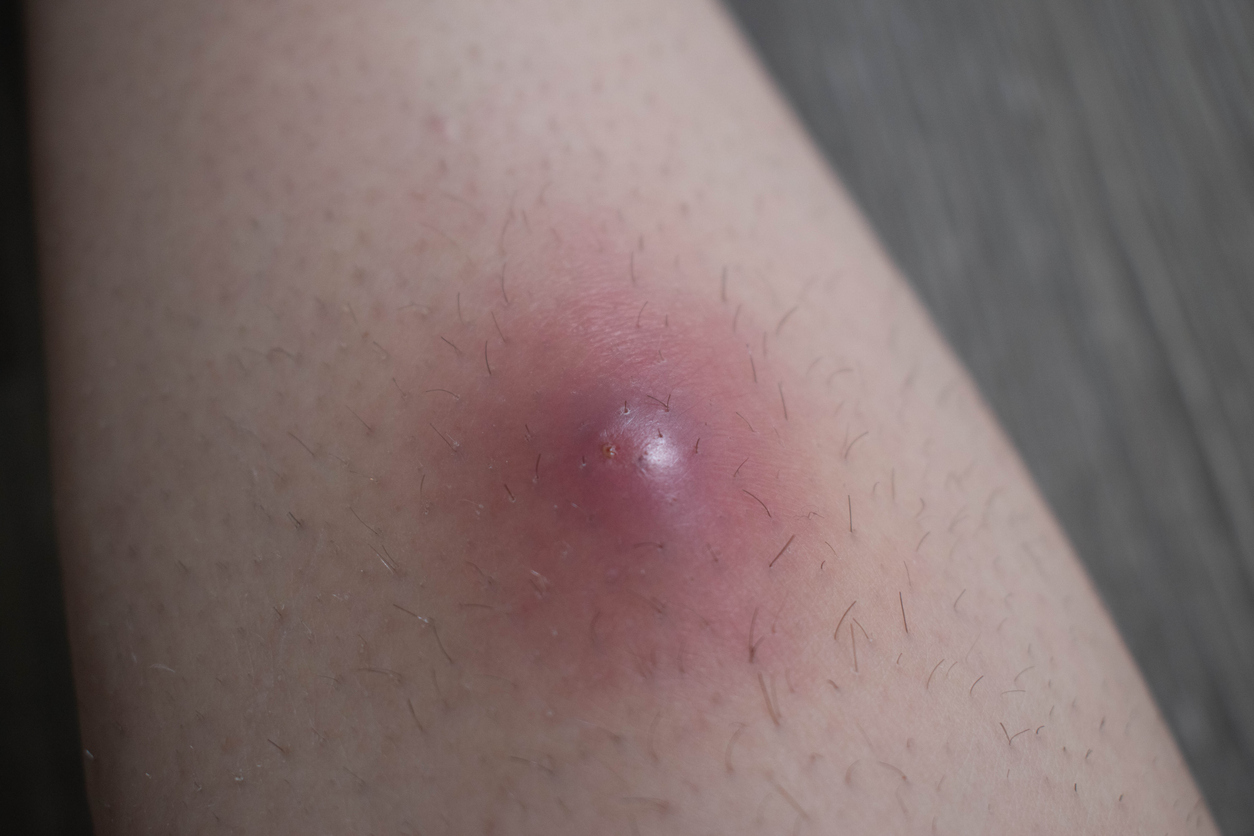
New Two-year Data: Bimekizumab Provides Sustained Disease Control in HS
February 13, 2025

A Future Physician’s Journey With HS
December 2, 2024

Meeting Unmet Needs in HS With UCB’s Dr. Jeff Stark
November 25, 2024









