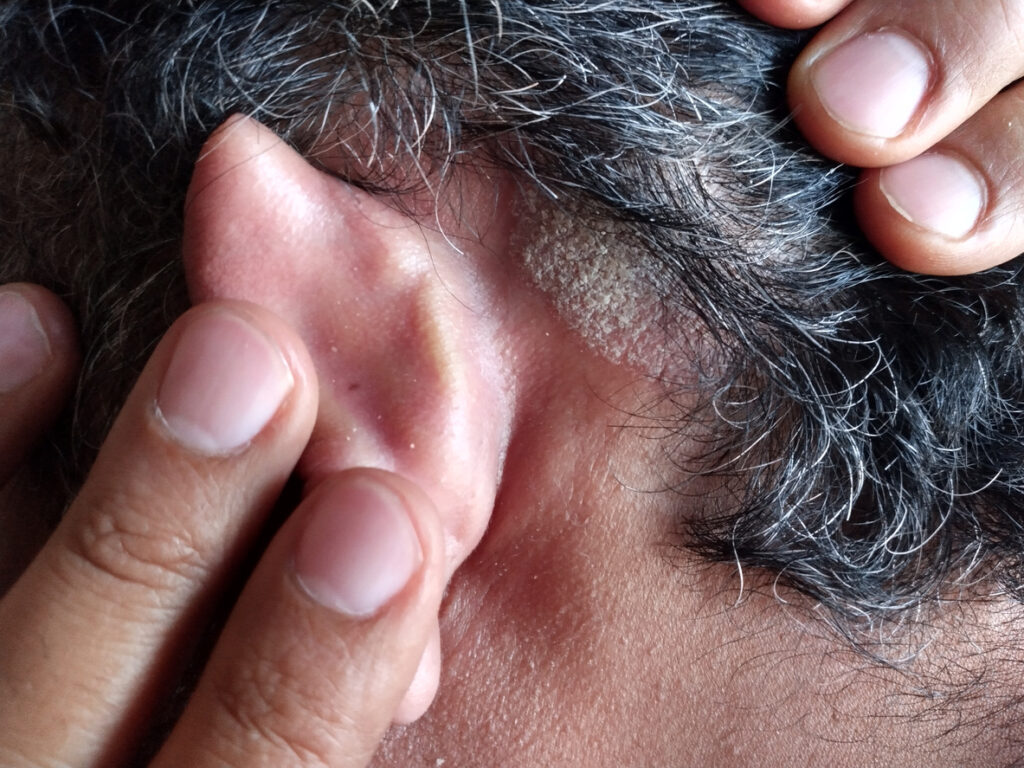
Health Canada has accepted Arcutis Canada, Inc.’s Supplement to a New Drug Submission (SNDS) for roflumilast foam 0.3% for review for the topical treatment of scalp and body psoriasis in patients aged 12 and up.
Roflumilast foam is a once-daily phosphodiesterase-4 (PDE4) inhibitor.
The SNDS is supported by data from Arcutis’ pivotal ARRECTOR (A Randomized tRial Employing topiCal roflumilasT foam to treat scalp psORiasis) Phase 3 trial, a Phase 2b study, and long-term efficacy and safety data generated from the cream development program in plaque psoriasis.
Key findings from the ARRECTOR trial include:
- Efficacy Outcomes: At Week 8, 66.4% of individuals treated with roflumilast foam achieved Scalp-Investigator Global Assessment (S-IGA) success, defined as a score of ‘clear’ or ‘almost clear’ with at least a two-point improvement from baseline, compared to 27.8% of individuals treated with vehicle. Similarly, 45.5% of participants achieved Body-Investigator Global Assessment (B-IGA) success with roflumilast foam versus 20.1% with vehicle.
- Itch Reduction: Clinically meaningful improvements in itch were observed, with 65.3% of individuals experiencing at least a 4-point reduction in the Scalp Itch Numeric Rating Scale (SI-NRS) compared to 30.3% with vehicle. Some patients reported noticeable relief within 24 hours of the first application. In addition, improvement in body itch as measured by the Worst Itch-Numeric Rating Scale (WI-NRS) was also observed at Week 8, with 63.1% of those treated with roflumilast foam achieving a ≥ 4-point reduction in WI-NRS compared to 30.1% of those treated with vehicle.
- Safety and Tolerability: Roflumilast foam was well tolerated across studies, with treatment-emergent adverse events (TEAEs) predominantly mild to moderate in severity. The most common adverse reactions (≥1%) included headache (3.1%), diarrhea (2.5%), nausea (1.7%), and nasopharyngitis (1.3%). Discontinuation rates due to adverse events were low and comparable to vehicle-treated patients.
Roflumilast cream 0.3% is approved in Canada for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Health Canada has authorized the use of roflumilast foam 0.3% for the treatment of seborrheic dermatitis in patients 9 and older. Roflumilast cream 0.15% is under review by Health Canada for the treatment of mild to moderate atopic dermatitis (AD) in adults and children ages 6 years and older, supported by clinical results from Arcutis’ Phase 2 and pivotal Phase 3 trials in AD.
In the U.S., roflumilast cream 0.15%, is indicated for topical treatment of mild to moderate atopic dermatitis in adult and pediatric patients aged 6 and older, roflumilast foam, 0.3% is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients aged 9 years and older. In addition, roflumilast cream, 0.3%, is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in adult and pediatric patients aged 6 and up.