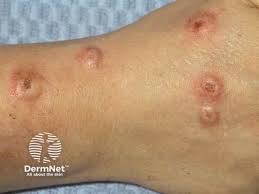
Just days after snagging dual approvals for moderate-to-severe atopic dermatitis (AD) and prurigo nodularis (PN) in the European Union, nemolizumab (Nemluvio, Galderma) was also granted Marketing Authorization in the United Kingdom (UK) and Switzerland for AD and PN.
The UK Medicines and Healthcare products Regulatory Agency and Swissmedic have granted the marketing authorization of nemolizumab for the treatment of both AD and PN in the UK and Switzerland, respectively.
Specifically, the approvals are for nemolizumab’s subcutaneous use for the treatment of moderate-to-severe AD in combination with topical corticosteroids and/or calcineurin inhibitors in adults and adolescents 12 years of age and older with a body weight of at least 30kg, who are candidates for systemic therapy, and for subcutaneous use for the treatment of adults with moderate-to-severe PN who are candidates for systemic therapy.
These are the first approvals from countries within the Access Consortium framework, an international collaborative initiative comprised of regulatory authorities which work together to address shared challenges. Decisions in the remaining countries within the Access Consortium, Australia and Singapore, are expected later this year. Nemolizumab is also approved in the European Union and United States for both moderate-to-severe AD and PN.
“These approvals offer patients with moderate-to-severe atopic dermatitis and prurigo nodularis a much-needed novel treatment option, given the potential burden and negative impact on quality of life associated with these conditions. I am looking forward to offering my patients this new treatment option which has the potential to address their most troublesome symptoms,” says Curdin Conrad, MD, a Professor of Dermatology and Head of Policlinic and the center for psoriasis at Lausanne University Hospital in Switzerland, in a news release.
This approval is based on robust results from the Phase III ARCADIA and OLYMPIA clinical trial programs, which demonstrated that nemolizumab clinically improved skin lesions, itch, and sleep disturbance in atopic dermatitis and prurigo nodularis, respectively. Nemolizumab was well tolerated in both clinical trial programs, and its safety profile was generally consistent with earlier data, and between trials. “Nemolizumab’s continued regulatory success underscores Galderma’s strong leadership in Therapeutic Dermatology, and our commitment to bringing innovative treatments to the patients,” says Baldo Scassellati Sforzolini, MD, PhD, Global Head of R&D at Galderma.
Nemolizumab, an interleukin (IL)-31 inhibitor is also approved by the U.S. Food and Drug Administration for the treatment of AD and PN.
PHOTO CREDIT: DermNet