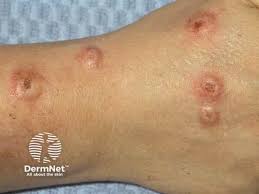
Patients with prurigo nodularis who were treated with nemolizumab (Nemluvio. Galderma) had significantly higher improvements in itch and skin lesions when compared to those receiving placebo at Week 16, with a rapid and clinically meaningful response on itch and sleep disturbance observed as early as Week 4, according to the Phase 3OLYMPIA 1 trial results.
Nemolizumab was well-tolerated, and its safety profile was generally consistent with previous studies.
The full results from the Phase 3 OLYMPIA 1 trial were published in JAMA Dermatology.
Nemolizumab is a monoclonal antibody that inhibits the signaling of interleukin (IL)-31, a neuroimmune cytokine that drives multiple disease mechanisms in prurigo nodularis.
The Phase 3 OLYMPIA 1 trial enrolled 286 adult patients with moderate-to-severe prurigo nodularis. Results demonstrated that patients treated with nemolizumab monotherapy (without background topical corticosteroids or topical calcineurin inhibitors) showed clinically meaningful and statistically significant improvements in both primary endpoints, compared to placebo. After 16 weeks of treatment, more than three times as many nemolizumab-treated patients:
- Achieved an at least four-point improvement in itch intensity, as measured by the peak-pruritus numerical rating scale (PP-NRS), when compared to the placebo group (58.4% vs 16.7%).
- Reached clearance or almost-clearance of skin lesions, when assessed using the investigator’s global assessment (score of 0 or 1 and a ≥2-point improvement from baseline), compared to the placebo group (26.3% vs 7.3%).
The trial also met all key secondary endpoints confirming rapid responses on itch and sleep disturbance as early as Week 4:
- More than six times as many nemolizumab-treated patients achieved itch response when compared to the placebo group (41.1% vs 6.3%), as measured by a four-point or greater reduction in PP-NRS score.
- More than twenty times as many nemolizumab-treated patients achieved a PP-NRS score of less than two, when compared to the placebo group (21.6% vs 1.0%). Results improved through to Week 16 (34.2% vs 4.2%).
- Almost six times as many nemolizumab-treated patients demonstrated a four-point improvement in sleep disturbance, as measured by the sleep disturbance numerical rating scale, when compared to the placebo group (31.1% vs 5.2%). Results improved through to Week 16 (50.0% vs 11.5%).
These full results reinforce previous findings from the Phase 3 OLYMPIA 2 trial, published in the New England Journal of Medicine, which demonstrated that nemolizumab rapidly and significantly improved itch and skin lesions in patients with prurigo nodularis, with clinically meaningful responses on itch observed as early as Week 4.
Based on data from the OLYMPIA clinical trial program, nemolizumab has been approved by the U.S. Food and Drug Administration for the treatment of adults with prurigo nodularis under the name Nemluvio. Galderma also has marketing authorization applications for nemolizumab in both prurigo nodularis and atopic dermatitis under review by multiple additional regulatory authorities, including the European Medicines Agency and via the Access Consortium framework in countries such as Australia, Singapore, and Switzerland, as well as Canada, Brazil, and South Korea. Submissions to regulatory authorities in additional countries are ongoing.
PHOTO CREDIT: DermNet