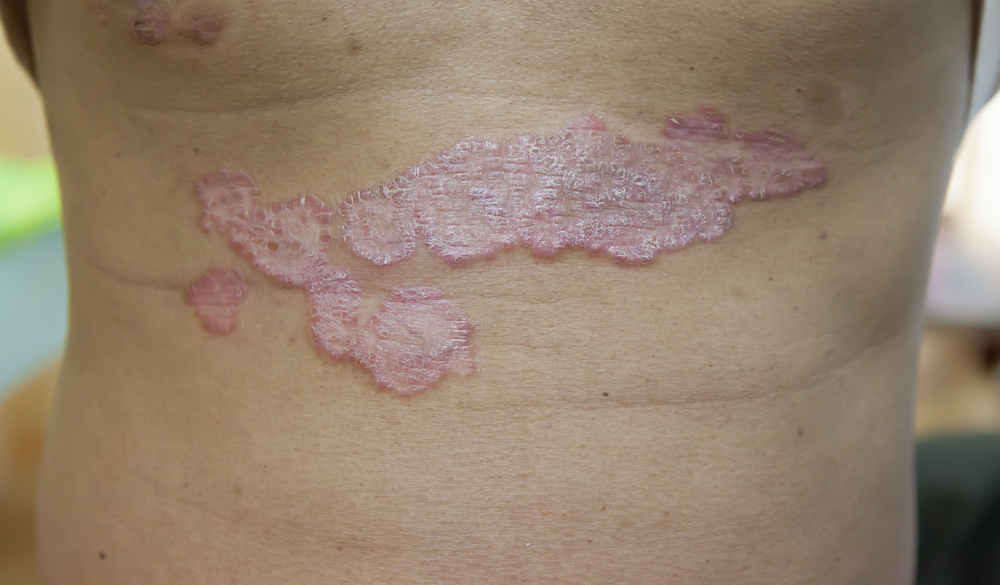
The first subject has been dosed in a Phase 1b trial evaluating VYNE Therapeutics Inc.’s VYN202 in moderate-to-severe plaque psoriasis.
VYN202 is an oral small molecule BD2-selective bromodomain and extra-terminal domain (BET) inhibitor that is being developed for the treatment of immune-mediated diseases.
The Phase 1b trial is a randomized, double-blind, placebo-controlled trial evaluating the safety, tolerability and pharmacokinetics of once-daily oral VYN202 in three dosing cohorts (0.25mg, 0.5mg, 1mg doses), compared to placebo, for 12 weeks in subjects with moderate-to-severe plaque psoriasis. Exploratory efficacy of VYN202 will also be evaluated including measures of psoriasis area and severity index (PASI), Static Physician Global Assessment (sPGA), scalp disease, quality of life, and biomarker analyses. Subjects will be randomized equally (1:1:1:1 ratio) across the active drug cohorts or placebo (~20 subjects in each arm). Following the 12-week treatment period, all subjects will be followed for a 4-week safety period.
Top-line data from the 12-week randomized, placebo-controlled trial are expected by year-end 2025.
“The initiation of the Phase 1b trial in subjects with moderate-to-severe plaque psoriasis represents a major step forward in advancing our novel and highly selective oral BET inhibitor, VYN202,” says David Domzalski, President and Chief Executive Officer of VYNE, in a news release. “Psoriasis shares common underlying biological pathways with several other chronic inflammatory conditions, and we believe results from this trial will provide key insights into VYN202’s potential use as a novel, once-daily oral treatment for chronic immune-mediated diseases.”
“Our Phase 1a single ascending dose (SAD) and multiple ascending dose (MAD) trial showed that VYN202 had a favorable safety profile and also demonstrated VYN202’s potential to inhibit the production of multiple inflammatory biomarkers related to Th17, TNF and Th1/myeloid dysregulated activity,” adds Iain Stuart, PhD, Chief Scientific Officer of VYNE. “We look forward to reporting results from this robust Phase 1b trial by the end of this year.”