Patients with advanced basal cell carcinoma (BCC) may benefit from receiving immunotherapy earlier in the course of treatment, according to a new study presented at the European Society for Medical Oncology (ESMO) annual meeting in Berlin, Germany.
First-line nivolumab (Opdivo, BMS), a programmed death 1 (PD-1) immune checkpoint inhibitor, produced an objective response rate (ORR) of 52% in 29 patients with inoperable BCC. In this study, the ORR is significantly higher than published response rates of approximately 25%-30% for second-line anti-PD-1 administered after a hedgehog pathway inhibitor, the current standard of care, the researchers report.
“Our results show that we can improve the likelihood of tumor response in patients with advanced basal cell carcinoma by administering anti-PD-1 in the front line, rather than after hedgehog pathway inhibitors. We look forward to further investigating these findings in larger, randomized trials,” says Govind Warrier, MD, MPH, Assistant Professor of Oncology at the Johns Hopkins Kimmel Cancer Center, Bloomberg~Kimmel Institute for Cancer Immunotherapy in Baltimore, MD, and a co-leader of the study. “These findings highlight important opportunities to improve outcomes for patients with advanced BCC, a population with few effective treatments.”
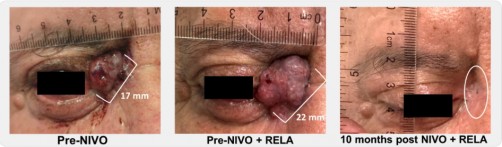
Investigators also explored whether adding relatlimab (Opdualag,BMS), an anti–LAG-3 immune checkpoint inhibitor, to nivolumab could induce tumor regressions in patients whose disease had progressed on nivolumab alone. Among 13 such patients, the ORR was 31% (4 of 13). The rationale for testing this combination was based on promising results in melanoma studies, where adding anti-LAG-3 to anti-PD-1 helped overcome resistance to single agent anti-PD-1, and from work at Johns Hopkins revealing that LAG-3 may be an important immune checkpoint in BCC.
“We discovered that LAG-3 is commonly expressed in the tumor microenvironment of aggressive basal cell carcinomas, suggesting LAG-3 blockade could be an attractive therapeutic option for these patients,” adds Julie Stein Deutsch MD, Assistant Professor of Dermatology, Pathology, and Oncology at the Johns Hopkins University School of Medicine, member of the Bloomberg~Kimmel Institute for Cancer Immunotherapy, and a co-leader of the study. (LAG-3 was first co-characterized by scientists at the Bloomberg–Kimmel Institute for Cancer Immunotherapy.)
PHOTO CAPTION: Durable, ongoing near-complete response to nivolimab + relatlimab in a 64-year-old with locally-advanced BCC after resistance to 26 weeks of nivolimab alone.

