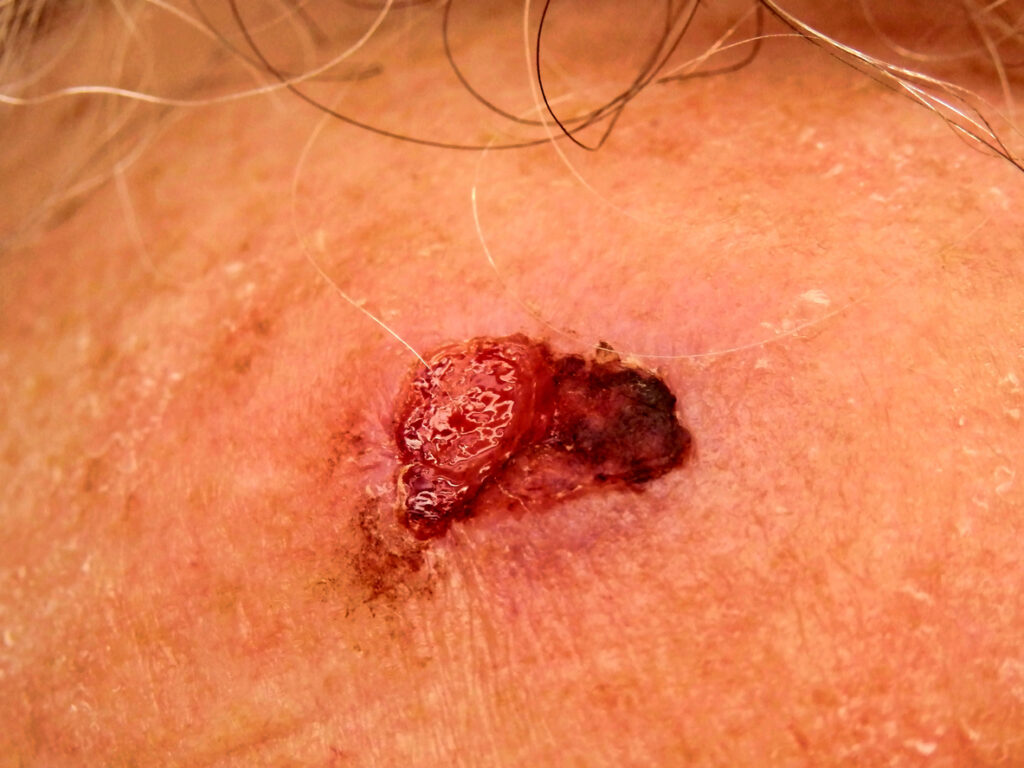
The U.S. Food and Drug Administration (FDA) has approved Alpha Tau Medical Ltd.’s Investigational Device Exemption (IDE) application to initiate a multi-center study for the treatment of recurrent cutaneous Squamous Cell Carcinoma (cSCC) in immunocompromised patients using Diffusing Alpha-emitters Radiation Therapy (Alpha DaRT).
Alpha DaRT is designed to enable highly potent and conformal alpha-irradiation of solid tumors by intratumoral delivery of radium-224 impregnated sources. When the radium decays, its short-lived daughters are released from the sources and disperse while emitting high-energy alpha particles with the goal of destroying the tumor. Since the alpha-emitting atoms diffuse only a short distance, Alpha DaRT aims to mainly affect the tumor, and to spare the healthy tissue around it.
The clinical study, which is an investigator-initiated study led by the Winship Cancer Institute of Emory University in Atlanta, GA, has been approved to enroll up to 28 U.S. patients at up to eight institutions in the U.S., and will focus on patients with recurrent cSCC who have a weakened immune system due to any primary or secondary immunodeficiencies, excluding diabetes. The primary efficacy objective of the study is the objective response rate (ORR) to the treatment, as measured by best overall response. Secondary efficacy objectives include progression-free survival, overall survival and local control up to twelve months after treatment, and the safety objective is the measurement of any related adverse events.
“As we continue to progress in our ReSTART multi-center pivotal trial for recurrent cutaneous SCC, a number of investigators asked about the ability to treat immunocompromised patients, who are ineligible for the ReSTART trial,” says Alpha Tau CEO Uzi Sofer, in a news release. “Emory University is an important partner of ours and we are proud to work with them in initiating a trial for this population. Given the continued requests we receive from clinicians to help them treat immunocompromised patients, we are confident that a successful clinical trial can help deliver an important new potential alternative for these patients.”
Zachary Buchwald, MD, PhD, an Assistant Professor of Radiation Oncology at Winship Cancer Institute of Emory University and Principal Investigator of the trial, adds, “We are thrilled to be able to initiate this trial and pioneer the use of the Alpha DaRT in immunocompromised patients. As we continue to see the promise of the Alpha DaRT through our participation in the ReSTART trial, it is obvious to us that we need to use this treatment elsewhere, particularly in populations at risk such as this.”
Dr. Robert B. Den, Alpha Tau Chief Medical Officer, agrees, “This trial targets a particularly vulnerable population for whom treatment options are limited, which affords the ability to provide even more potential value to these patients. We are looking forward to treating patients in this trial soon.”