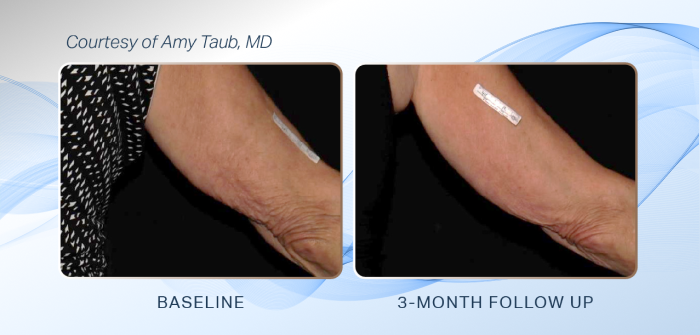
The U.S. Food and Drug Administration (FDA) has cleared Sofwave for the improvement of the appearance of skin laxity on the upper arms.
In the US, Sofwave’s SUPERB (Synchronous Ultrasound Parallel Beam) technology is currently cleared for use as a non-invasive aesthetic treatment to improve facial lines and wrinkles, lift the eyebrow, and lift lax submental area and neck tissue in people aged 22 and older. The Sofwave system is also cleared for short-term improvement in the appearance of cellulite and for the treatment of acne scars.
The study leading to the new clearance for upper arms included 46 people who were treated on both upper arms at four sites in the United States.
People attended two treatment sessions (1-3 weeks apart) and a follow-up visit three months after the final treatment visit. Fully 93% of treated arms were “improved” or “very much improved” in appearance, as rated by the blinded reviewers using the Global Aesthetic Improvement Scale.
Most people reported none to mild levels of pain during treatment and no discomfort afterward.
“I am so excited for Sofwave’s new FDA indication for loose upper arm skin! This amazing no-downtime technology never disappoints!,” Quenby Erickson, DO, founder of Erickson Dermatology and Lifestyle Medicine in Chicago, tells TDD. “It is not surprising that 93% of treated arms showed significant improvement in skin laxity rated by blinded reviewers. I have been using this technology for two years in my office, and patients are very happy!”