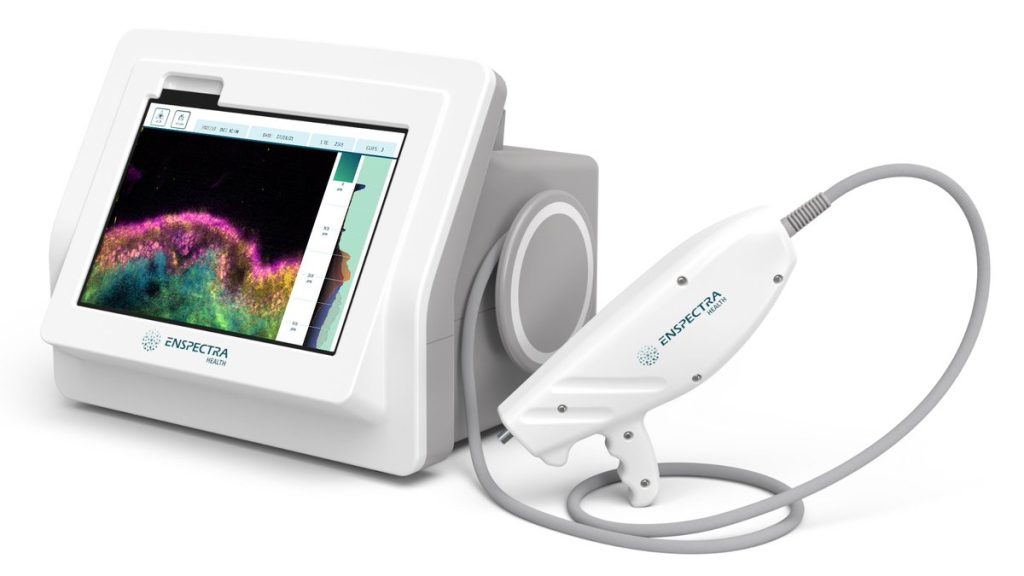
The U.S Food and Drug Administration (FDA) has granted 510(k) marketing clearance to Enspectra Health’s VIO System, a first-of-its-kind technology that combines reflectance confocal and multiphoton microscopy to enable cellular resolution, and cross-sectional imaging of skin in seconds.
Vio is portable, handheld, and digitizes pathology directly from a patient’s skin to provide clinical insights for physicians.
“We are thrilled to receive FDA clearance for the VIO System. This is the first multiphoton imaging modality to ever be cleared by the FDA,” says Gabriel Sanchez, PhD, CEO and co-founder of Enspectra Health, in a news release. “Our submission was cleared by the FDA ahead of plan, underscoring our ability to successfully drive an innovative technology from concept to clearance.”
The VIO System was evaluated in the prospective, multicenter VISTA US Pivotal Study, demonstrating that it is safe and effective in a practical clinical environment. The technology captures in vivo images of tissue – including blood vessels, collagen, pigment, stratum corneum, hair shafts or follicles, solar elastosis, hyperkeratosis, atypia, and epidermal disarray – in and through the epidermis, to assist physicians in forming a clinical judgment. VIO images are intended to be interpreted by dermatologists or pathologists with skin histology training. Physicians in the clinical study were able to identify skin features on VIO images with >90% average accuracy, exceeding study endpoints.
Limited systems will be made available to select clinical partners. Enspectra Health plans to pursue additional indications and further advance its AI/ML clinical decision support algorithms.
“The VIO System has tremendous potential for evaluating skin in clinical practice,” said Manu Jain, MD, a pathologist-scientist at Memorial Sloan Kettering Cancer Center in New York City. “The cross-sectional images from VIO can be used to inform clinical decision-making in a variety of dermatological conditions in real-time which can have a powerful impact on clinical workflow and the patient journey.”