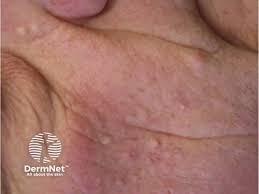
The U.S. Food and Drug Administration (FDA) has cleared Azitra, Inc.’s investigational new drug (IND) application for a first-in-human Phase 1/2 clinical study of ATR-04 for moderate to severe epidermal growth factor receptor inhibitor (EGFRI)-associated dermal toxicity.
ATR-04 is a live biotherapeutic product candidate including an isolated, naturally derived Staphylococcus epidermidis strain that was engineered to be safer by deleting an antibiotic resistance gene and engineering auxotrophy to control the growth of ATR-04.
ATR-04 is in development for EGFRi-associated skin rash, which is caused by the suppression of skin immunity by EGFRis and subsequent inflammation and often elevated levels of IL-36γ and S. aureus. There are approximately 150,000 patients suffering from EGFRi-induced skin toxicity in the United States.
Following the clearance of the IND, Azitra plans to initiate a multicenter, randomized, controlled Phase 1/2 clinical trial of ATR-04 in patients undergoing EGFR inhibitors with dermal toxicity by the end of 2024.
Earlier this year, Azitra announced the preclinical data around ATR-04 at the Society for Investigative Dermatology (SID) annual meeting and the Annual Meeting for the American Society of Cell and Gene Therapy, showing significant reductions in IL-36γ and methicillin-resistant S. aureus (MRSA) in preclinical models.
“Many cancer patients receive EGFR inhibitors, which are efficacious for certain cancers,” says Francisco Salva, Azitra’s CEO, in a news release. “However, these EGFR inhibitors often have significant side effects, resulting in rashes that require treatment with antibiotics, steroids or other medications. In some cases, discontinuation of cancer therapy with the EGFR inhibitors is necessary. There are no approved therapies for this skin toxicity, and it is a high burden for these cancer patients. We are excited to expand Azitra’s clinical pipeline with this IND.”
PHOTO CREDIT: DermNet