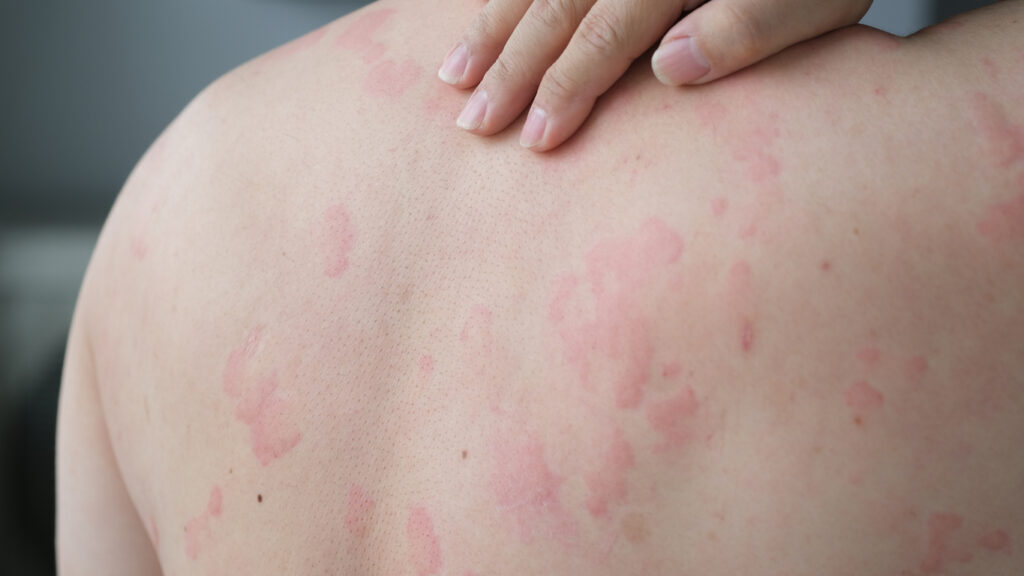
Add chronic spontaneous urticaria (CSU) to the list of U.S. Food and Drug Administration (FDA) approvals for dupilumab (Dupixent, Sanofi & Regeneron).
Dupilumab is now FDA-approved for its latest indication: treating patients aged 12+ years with uncontrolled CSU who remain symptomatic despite H1-antihistamines. Dupilumab is first targeted therapy for CSU in a decade.
This approval is supported by the CUPID clinical trial program, which demonstrated clinically meaningful symptom improvements in itch, hives, and overall disease control.
The LIBERTY-CUPID Phase 3 study program evaluating dupilumab for CSU consists of Study A, Study B, and Study C. Study A and Study C were conducted in CSU patients who were uncontrolled on standard-of-care antihistamines while Study B was conducted in CSU patients who were uncontrolled on standard-of-care antihistamines and refractory or intolerant to omalizumab.
The resubmitted sBLA adds results from Study C, which was conducted in patients with uncontrolled CSU who were on standard-of-care antihistamines. Study C met its primary and key secondary endpoints, confirming results seen in the previous Study A. Results showed Dupixent significantly reduced itch and urticaria activity.
Safety results in all LIBERTY-CUPID phase 3 studies were generally consistent with the known safety profile of dupilumab in its approved indications. Adverse events more commonly observed with dupilumab t (≥5%) compared to placebo were injection site reactions and COVID-19 infection.
Dupilumab has been approved for CSU in Japan and the United Arab Emirates (UAE) and is also under regulatory review in the EU based on earlier study readouts. Dupilumab is approved for certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, prurigo nodularis, and chronic obstructive pulmonary disease in different age populations.