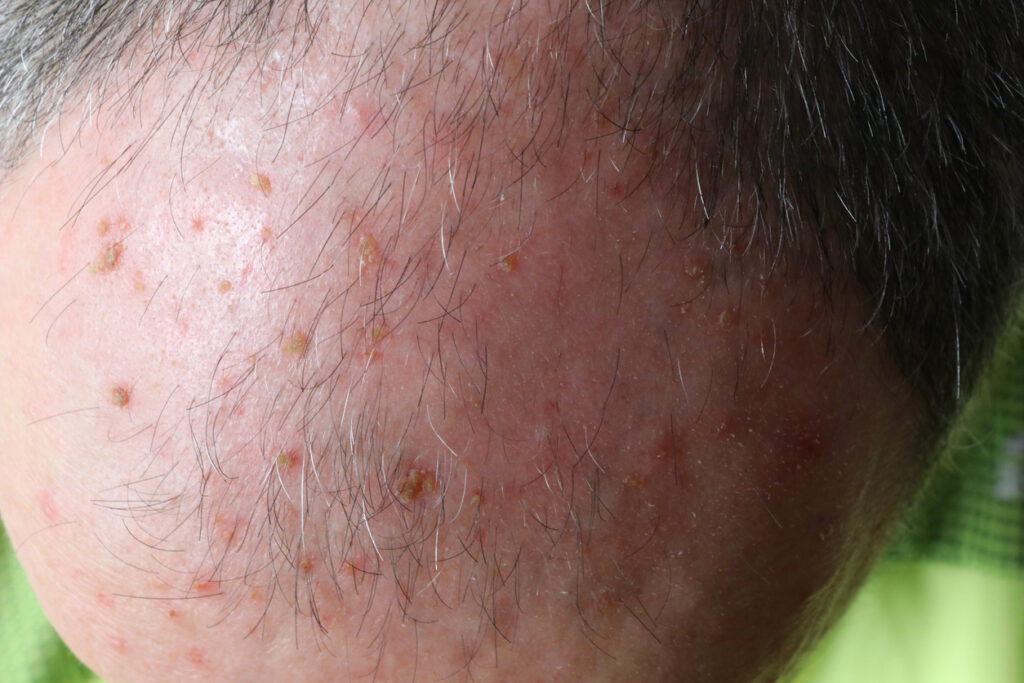
The U.S. Food and Drug Administration (FDA) approved Almirall’s recent supplemental New Drug Application (sNDA) to expand the use area of tirbanibulin (Klisyri) for the treatment of actinic keratosis (AK) on the face or scalp up to 100 cm2.
Klisyri, a microtubule inhibitor ointment, is now approved in a 350 mg package size and is a 5-day topical field treatment for actinic keratosis (AK) of the face or scalp. Klisyri will now be available in two package sizes, 250 mg (NDC 16110-391-05) for the treatment of up to 25 cm2, and 350 mg (NDC 16110-391-55)] for up to 100 cm2.
“The FDA’s approval of the use of Klisyri for actinic keratosis on an extended surface of the face or scalp is a significant step forward for both patients and treating dermatologists. With patients experiencing AK over larger surface areas, dermatologists are looking for ways to treat the entire affected area to help prevent further lesion progression,” saysKarl Ziegelbauer, Chief Scientific Officer at Almirall, in a news release.
This new approval will change the previous Klisyri dosing for surface area treatment from up to 25 cm2 to up to 100 cm2, allowing clinicians to treat a larger area of the face or balding scalp. The sNDA was supported by an additional Phase 3, multicenter, open-label, clinical safety study with more than 100 patients in the US. The primary endpoints of the study were to evaluate the safety and tolerability of applying tirbanibulin to a field of approximately 100 cm2 on the face or balding scalp of adult AK patients. The study showed consistent results with the original pivotal trials conducted on an area of 25 cm2, for both local skin reactions and treatment related adverse events (AEs).
The effectiveness of tirbanibulin in a larger treatment area was also explored, showing a percent reduction in AK lesion count in line with the one reported in the original pivotal studies.
“With this new FDA approval, clinicians can now treat up to four times the surface area, allowing increased flexibility to provide treatment of actinic keratoses and achieve effective results with a good safety and tolerability profile for more patients,” says Neal Bhatia, MD, a dermatologist in San Diego, CA, who served as the principal investigator for the larger treatment area pivotal study.