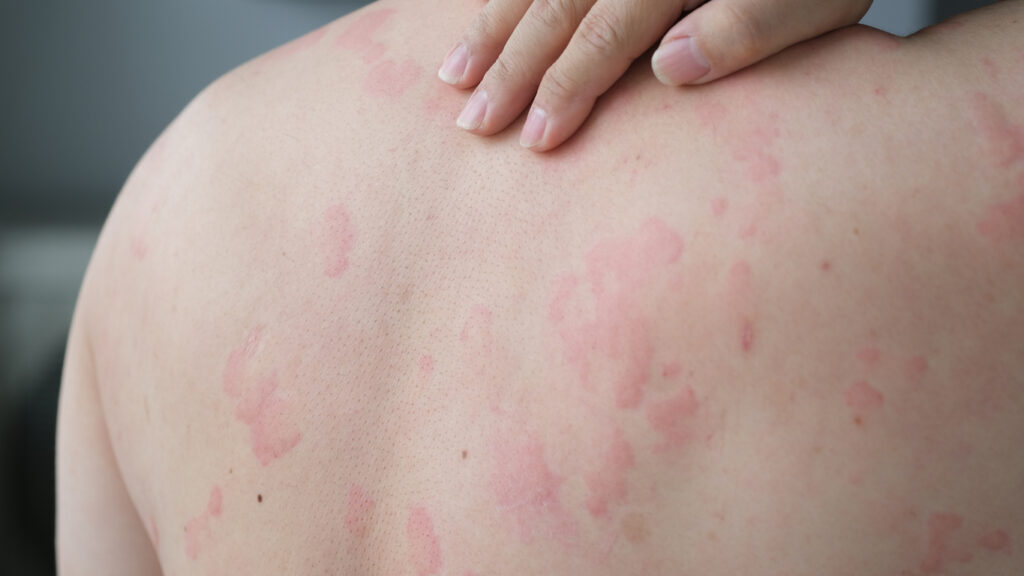
The U.S. Food and Drug Administration (FDA) has accepted for review the resubmission of the supplemental Biologics License Application (sBLA) for dupilumab (Dupixent, Sanofi & Regeneron) to treat adults and pediatric patients aged 12 years and older with chronic spontaneous urticaria (CSU) whose disease is not adequately controlled with H1 antihistamine treatment.
The new Prescription Drug User Fee Act (PDUFA) target action date for the FDA decision is April 18, 2025. If approved, dupilumab would be the first targeted therapy for CSU in a decade.
The resubmitted sBLA is supported by data from the multi-study LIBERTY-CUPID phase 3 clinical program (Study A, Study B, and Study C) for dupilumab in CSU, which confirm dupilumab significantly reduced itch and hive activity. Safety results in all LIBERTY-CUPID phase 3 studies were generally consistent with the known safety profile of dupilumab.