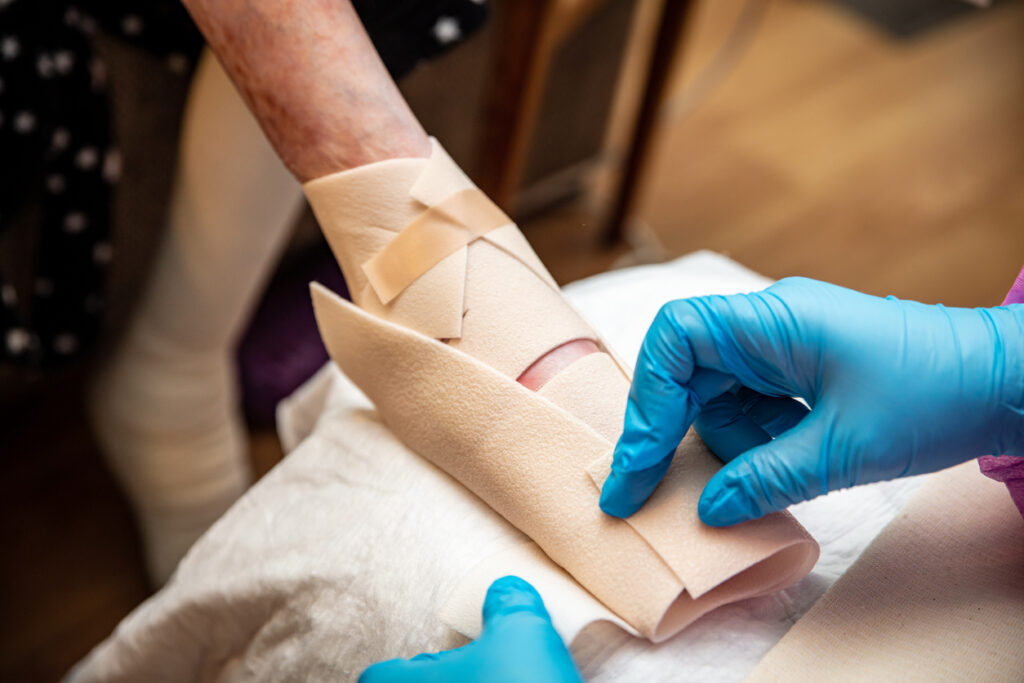
The U.S. Food and Drug Administration (FDA) has accepted Abeona’s resubmission of its Biologics License Application (BLA) for prademagene zamikeracel (pz-cel) for the treatment of recessive dystrophic epidermolysis bullosa.
Pz-cel is an investigational autologous cell-based gene therapy.
The FDA has assigned a Prescription Drug User Fee Act (PDUFA) target action date of April 29, 2025.
“The FDA acceptance of our BLA resubmission moves us one step closer to providing pz-cel as a differentiated treatment option to address the persistent unmet needs of people with RDEB in the U.S.,” says Vish Seshadri, Chief Executive Officer of Abeona, in a news release. “We look forward to continuing to work with the FDA to finalize the review of the pz-cel application.”
The BLA resubmission is supported by clinical efficacy and safety data after a one-time administration of pz-cel from the pivotal Phase 3 VIITAL study (NCT04227106) and a Phase 1/2a study (NCT01263379) with up to eight years of follow-up. If approved, pz-cel would be the first autologous, cell-based gene therapy for RDEB, and the first RDEB treatment designed to provide collagen VII expression at wound sites via a stably integrated copy of the COL7A1 gene.
The Company’s BLA for pz-cel was previously accepted for Priority Review by the FDA for patients with RDEB. Abeona may be eligible for a Priority Review Voucher (PRV) should pz-cel be approved.