New research highlights the safety, tolerability, and antitumor effects of Verrica Pharmaceuticals Inc.’s P-315 in basal cell carcinoma.
VP-315 is a novel oncolytic peptide under development for the treatment of basal cell carcinoma (BCC). The positive preliminary topline results from Part 2 of a Phase 2 study will be presented at Fall Clinical Dermatology Conference in Las Vegas, NV.
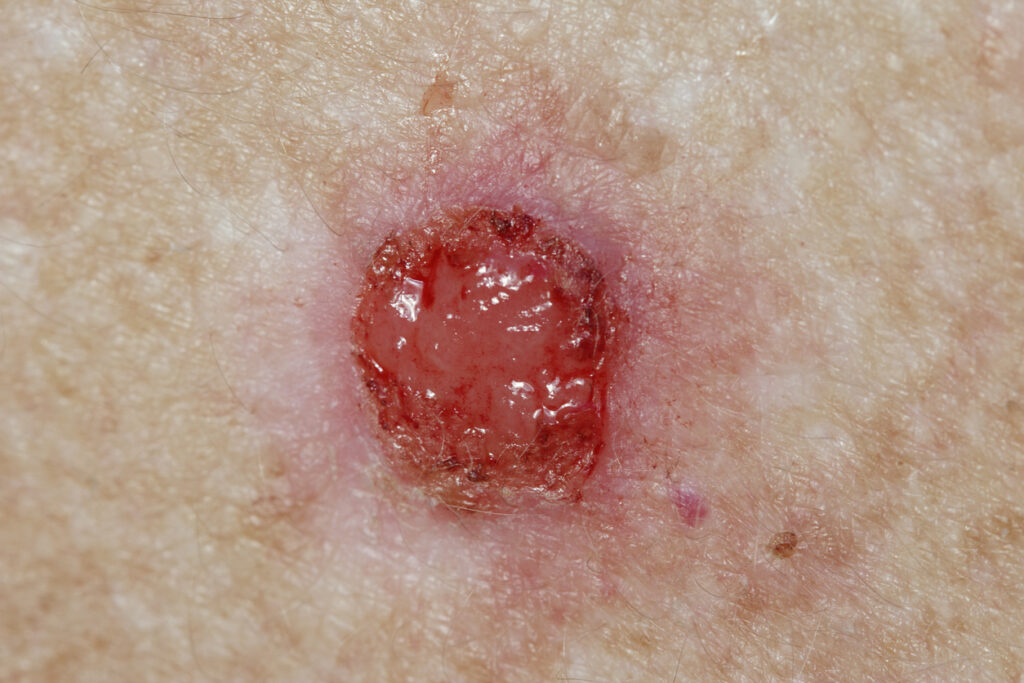
The new posters include safety and histologic clearance data from 82 patients with up to 2 target BCC tumors (total 92 tumors), including patients with tumors on the head and neck.
Approximately 51% of tumors treated in Part 2 achieved complete histological clearance and patients with residual tumor on average achieved approximately 71% reduction in tumor size.
There were no Treatment Related Serious Adverse Events, and most Treatment Related Adverse Events were mild to moderate.
Part 2 of the Phase 2 trial was designed to further explore dosing regimens to help identify the recommended regimen for a Phase 3 study program. The Company expects genomic and T-cell (immune response) data in the first quarter of 2025 and plans to request an End-of-Phase 2 meeting with the U.S. Food and Drug Administration to determine the next steps for the development of VP-315 for the treatment of BCC in the first half of 2025.
“We believe the positive preliminary topline results from Part 2 of the Phase 2 study for VP-315 are a meaningful step forward in potentially providing BCC patients with additional treatment options,” says Ted White, President and Chief Executive Officer of Verrica, in a news release. “We are encouraged by our preliminary results, which we believe support the use of VP-315 as a first line therapy for use in both primary, and neoadjuvant settings. As a novel oncolytic peptide administered directly into the tumor, VP-315 has the potential to offer a non-surgical alternative for the three to four million cases of BCC diagnosed in the U.S. each year, representing a multi-billion-dollar commercial opportunity for Verrica.”
About the Phase 2 Trial of VP-315
The Phase 2 trial is a 2-part, open-label, multicenter, dose-escalation, proof-of-concept study with a safety run-in designed to assess the safety, pharmacokinetics, and efficacy of VP-315 when administered intratumorally to adults with biopsy-proven BCC. The study enrolled 92 adult subjects with a histological diagnosis of BCC in at least one eligible target lesion.