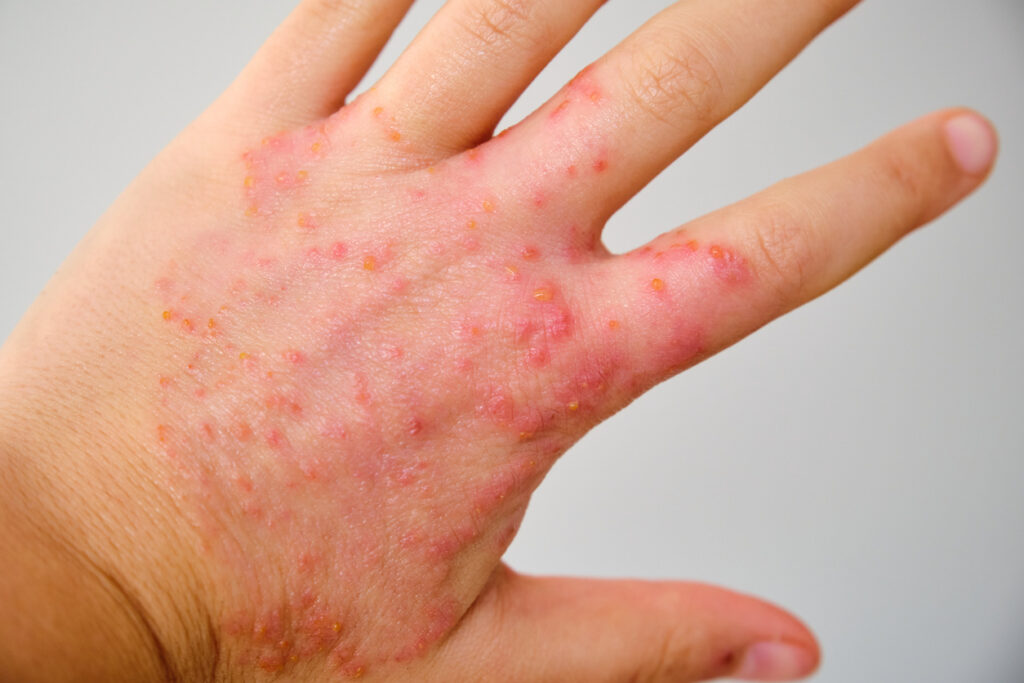
Fully 9.6% of people have a self-reported physician-diagnosed case of chronic hand eczema, according to new data from the CHECK (Chronic Hand Eczema epidemiology, Care, and Knowledge) study.
Of those who received a CHE diagnosis, 65.1% rated their disease as moderate to severe.
The new data, which were presented at the Fall Clinical Dermatology Conference in Las Vegas, NV, seek to quantify and qualify the toll that CHE takes on patients’ lives.
The CHECK study enrolled more than 10,000 adults aged 18 to 69. Notably, the data indicate a higher prevalence among men, employed participants, participants under 40 years of age, and residents of urban areas.
“These highly powered survey results provide compelling evidence that chronic hand eczema (CHE) is a common skin disease in the United States,” says Raj Chovatiya, Lead Investigator of the study and Associate Professor at Rosalind Franklin University of Medicine and Science Chicago Medical School in Chicago, IL, in a news release. “By deepening our understanding of its prevalence and identifying who is most likely to have CHE, we can better quantify the broader social and economic burden of this debilitating disease.”
One poster presented details of the severity, symptoms, and treatment of CHE from the CHECK study. The majority (65.1%) of those reporting CHE rated their disease as moderate to severe, and more than 80% were currently on systemic or topical treatment. However, despite treatment, CHE-related symptoms, including itch, pain, and sleep disturbances, persisted and were still commonly rated as moderate in severity, the survey showed.
The impact of CHE on occupation, work productivity, and activity impairment was also presented in a poster, demonstrating that a considerable proportion of respondents attributed their disease to their work (27.9%) or to common everyday activities (34.9%). Of those, many reported having to modify their activities, reduce their working hours, or even change their job, according to another poster.
The substantial negative impact of CHE on patients was supported by another poster that demonstrated there were significantly higher out-of-pocket costs per month for emollients or other topical treatments for patients with moderate-to-severe CHE compared to those with mild CHE.
Results from a national Ipsos survey of US healthcare professionals (HCPs) about their experience managing CHE were also presented. Among the surveyed HCPs (N=192), more than 90% strongly agreed that CHE impacts their patients’ ability to work and perform daily tasks, and 51% agreed that there is a lack of options available to effectively treat moderate-to-severe CHE.
The US Food and Drug Administration (FDA) approved delgocitinib cream (Anzupgo, LEO Pharma) for the topical treatment of moderate-to-severe CHE in adults who have had an inadequate response to, or for whom, topical corticosteroids are not advisable, in July 2025.
Delgocitinib cream inhibits the Janus kinase/signal transducer and activator of the transcription (JAK-STAT) pathway, specifically blocking the activity of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), and suppresses the various inflammatory responses that play a key role in the onset and subsequent flares of CHE.