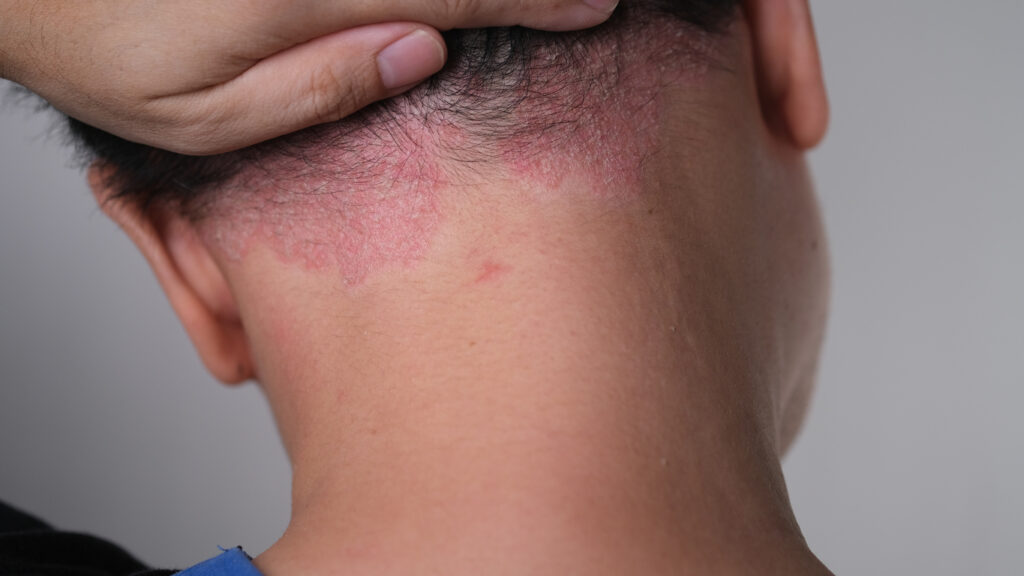
New long-term results further support the promise of once-daily oral icotrokinra in difficult-to-treat scalp and genital psoriasis.
Icotrokinra is a first-in-class investigational targeted oral peptide that precisely blocks the interleukin (IL)-23 receptor, in adults and pediatric patients aged 12 and older with plaque psoriasis (PsO) affecting high-impact sites.
The ICONIC-TOTAL study, which was presented at the 2025 Fall Clinical Dermatology Conference in Las Vegas, NV, simultaneously evaluated adults and adolescents with at least moderate scalp, genital, and/or hand/foot plaque psoriasis with ≥1% Body Surface Area (BSA) affected. Through Week 52, icotrokinra demonstrated high and durable rates of site-specific psoriasis clearance affecting all of these high-impact and difficult-to-treat areas of the body.
In areas of high impact, 72% of patients with scalp psoriasis and 85% with genital psoriasis treated with icotrokinra achieved site-specific Clear or Almost Clear skin at Week 52 in the Phase 3 ICONIC-TOTAL study. What’s more, 67% of patients treated with icotrokinra achieved overall rates of Clear or Almost Clear skin by Week 24, which was maintained through Week 52, the study showed.
In the smaller subset of patients with hand/foot psoriasis, treatment with icotrokinra showed a numerically higher rate of skin clearance at Week 16, which increased through Week 52 with patients achieving a hand and/or foot Physician’s Global Assessment (hf-PGA) score of 0/1 increasing from 42% to 62%.
“Many of the patients in my practice experience significant distress when psoriasis affects sensitive areas such as the scalp, genitals, hands, and feet,” says Edward (Ted) Lain, MD, MBA, Executive Director of the Austin Institute for Clinical Research in Austin, Texas, and study investigator, in a news release. “The durable response rates observed in the ICONIC-TOTAL study show that icotrokinra has the potential to be a meaningful new option for effectively managing moderate-to-severe plaque psoriasis long-term in both adults and adolescents.”
Overall response rates among patients treated with once daily icotrokinra were maintained through Week 52, with 67% of patients treated with icotrokinra achieving Clear or Almost Clear skin (Investigator’s Global Assessment (IGA) 0/1) and 44% achieving Completely Clear skin (IGA 0) at Week 52. The overall response rates were also comparable among patients who received icotrokinra for all 52 weeks and those who transitioned from placebo to icotrokinra at Week 16 (67% vs. 68% achieved IGA 0/1, respectively). Across treatment groups, adverse event and serious adverse event rates were similar through Week 52 compared to those through Week 16, with no new safety signals identified.
Icotrokinra was jointly discovered and is being developed pursuant to the license and collaboration agreement between Protagonist and Johnson & Johnson. Johnson & Johnson retains exclusive worldwide rights to develop icotrokinra in Phase 2 clinical trials and beyond, and to commercialize compounds derived from the research conducted pursuant to the agreement against a broad range of indications. Johnson & Johnson recently submitted an application to the European Medicines Agency (EMA) for what could be the first-ever approval of icotrokinra for the treatment of moderate-to-severe plaque psoriasis in people aged 12 and up.