The European Commission (EC) has granted marketing authorization for two 320mg device presentations of UCB’s bimekizumab (Bimzelx, UCB).
The pre-filled syringe and pre-filled pen each contain 320 mg of bimekizumab in a volume of 2mL and provide alternatives to the currently available 160 mg in a volume of 1 mL injection options. UCB developed the 320 mg single-injection options to increase convenience, simplify administration and enhance the individual patient experience, according to a Company-issued press release.
Bimekizumab is a humanized monoclonal IgG1 antibody designed to selectively inhibit both interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
The EC approval follows a positive opinion issued in May 2024 by the Committee for Medicinal Products for Human Use of the European Medicines Agency. The approval is based on data from studies evaluating the bioequivalence of bimekizumab 320mg given as one 2mL subcutaneous injection, and bimekizumab 320mg given as two 1mL subcutaneous injections, in healthy study participants.
Patients requiring a 320mg dose of bimekizumab will now have an alternative single-injection option of both the pre-filled syringe and the pre-filled pen
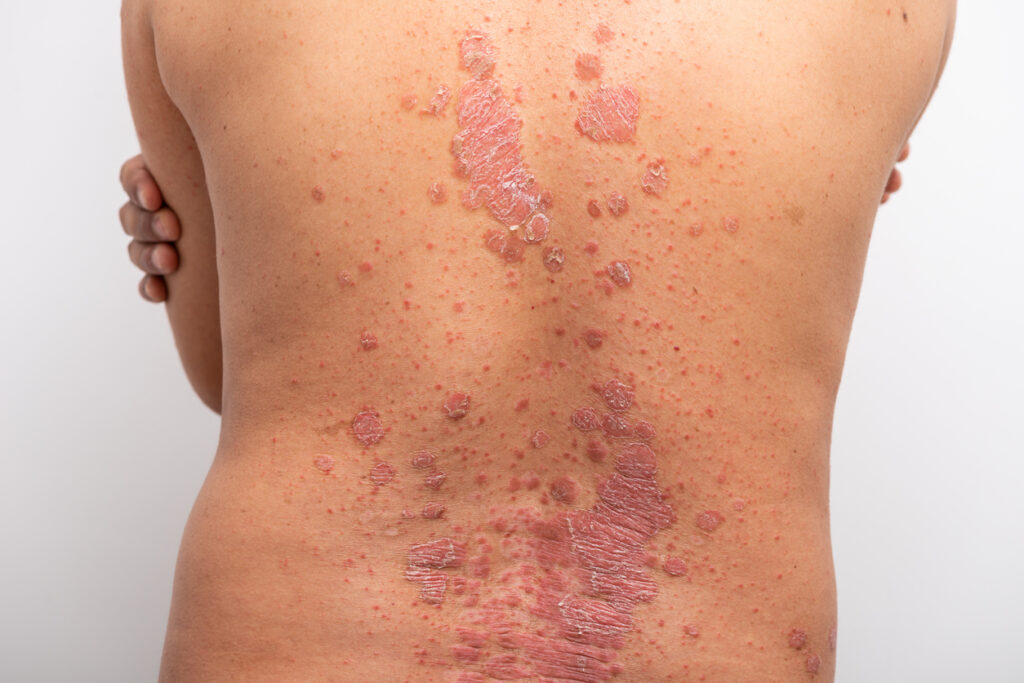
In the European Union, the indications for bimekizumab where a 320 mg dose is recommended are adults with moderate to severe plaque psoriasis, adults with active psoriatic arthritis with coexistent moderate to severe plaque psoriasis, and adults with active moderate to severe hidradenitis suppurativa.
Regulatory applications for the 320mg bimekizumab in a volume of 2mL device are currently in review with the U.S. Food and Drug Administration (FDA) and the Pharmaceuticals and Medical Devices Agency in Japan.