Alumis Inc. and Kaken Pharmaceutical Co., Ltd. have entered into a collaboration and licensing agreement to develop, manufacture, and commercialize ESK-001, a highly selective, next-generation oral tyrosine kinase 2 (TYK2) inhibitor, for dermatology indications in Japan, with the option to expand the license to include rheumatological and gastrointestinal diseases.
Under the terms of the agreement, Alumis will receive $40 million in upfront and near-term co-development payments in 2025 to 2026, with the potential to earn up to approximately $140 million in additional payments based on the achievement of milestones, and field option payments. Alumis is also eligible to receive tiered royalties ranging from the low double-digits into the twenties on aggregate net sales of ESK-001 in Japan.
Kaken will be responsible for the clinical development, regulatory approvals, and commercialization of ESK-001 in Japan, and Alumis will retain rights to ESK-001 in all other geographies. Kaken will also contribute to a portion of the global development costs of ESK-001.
Learn more about ESK-001 in psoriasis with Dr. Andrew Blauvelt here.
“We are thrilled to announce this agreement with Kaken, a dermatology leader with significant reach and expertise in the Japanese market,” says Martin Babler, President and Chief Executive Officer of Alumis, in a news release. “This partnership builds on the positive Phase 2 clinical data of ESK-001, our next-generation oral TYK2 inhibitor, supporting our objectives to unlock its full therapeutic potential and ensure ESK-001 is widely accessible to people with immune-mediated disorders around the world.”
“We are delighted to enter into an agreement with Alumis to develop ESK-001 for the Japanese market,” adds Hiroyuki Horiuchi, President and Representative Director of Kaken. “We strongly believe in the potential of ESK-001 to address a range of medical needs in the dermatology space and potentially rheumatological and gastrointestinal diseases in the future. ESK-001 will be an important addition to the Kaken portfolio of novel therapeutics for dermatology conditions.”
More About ESK-001
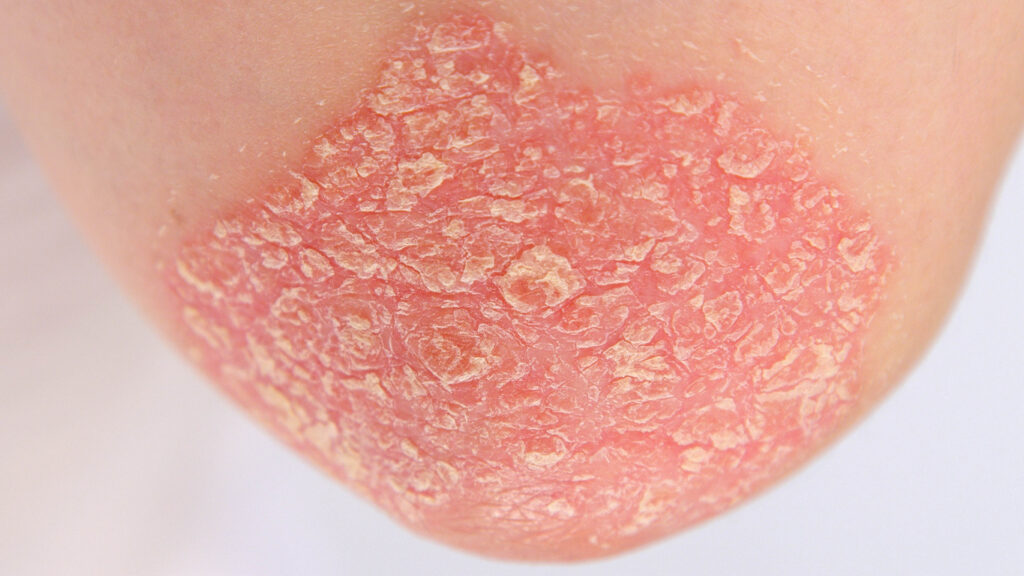
ESK-001 is currently being evaluated in the Phase 3 ONWARD clinical program, which consists of two parallel global Phase 3, multi-center, randomized, double-blind placebo-controlled 24-week clinical trials, ONWARD1 and ONWARD2, including trial sites in Japan, designed to evaluate the efficacy and safety of ESK-001 in adult patients with moderate-to-severe plaque psoriasis.
Patients completing Week 24 will have the opportunity to participate in a long-term extension (LTE) trial, ONWARD3, that will evaluate durability and maintenance of response and long-term safety. The Phase 3 clinical program is supported by positive data from the Phase 2 STRIDE clinical trial and the ongoing long-term OLE extension showing that ESK-001 treatment led to robust long-term clinical responses and was well tolerated through 52 weeks. In parallel with the Phase 3 clinical program, Alumis is developing a once-daily modified release oral formulation of ESK-001 designed to replace the current immediate release oral formulation that is dosed twice daily.
ESK-001 is also being evaluated in LUMUS, a Phase 2b clinical trial for the treatment of patients with systemic lupus erythematosus. In addition, Alumis continues to leverage its precision data analytics platform to explore ESK-001’s potential application in other immune-mediated conditions.