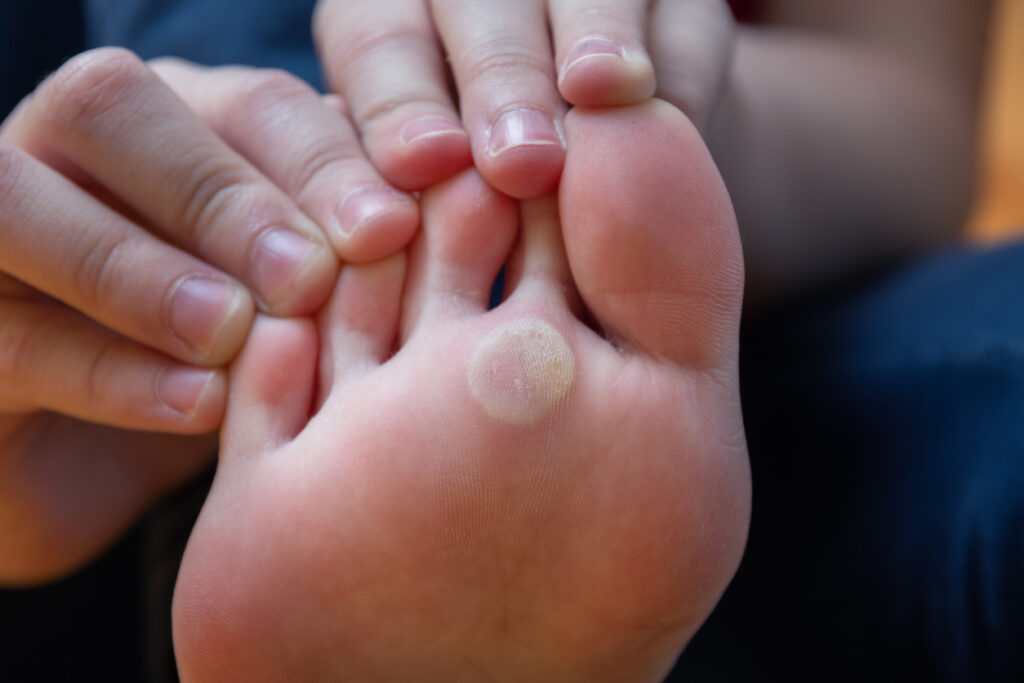
The final patient has been successfully enrolled in Nielsen BioSciences, Inc.’s CFW-3A, a Phase 3, randomized, double-blind, placebo-controlled study of the safety and rfficacy of Candin (Candida albicans Skin Test Antigen for Cellular Hypersensitivity) for the treatment of common warts (Verruca vulgaris) in adolescents and adults.
Candin is currently marketed in the United States for its U.S. Food and Drug Administration-approved use as a skin test antigen for the assessment of cellular hypersensitivity to Candida albicans. Candin is not currently approved for the treatment of common warts.
“I am pleased that the study accomplished this important milestone. Candida albicans has been well studied, but this is the first time that a randomized controlled Phase 3 clinical study to investigate safety, efficacy, and optimal dosing for the treatment of warts has been conducted,” says Sandra Johnson, MD, FAAD, Dermatologist from Fort Smith Arkansas and one of the principal investigators of this clinical trial. “We look forward to the conclusion and analysis of this data to hopefully improve the treatment of common warts in adolescents and adults.”
The study is a Phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of Candin (Candida albicans Skin Test Antigen for Cellular Hypersensitivity) for the treatment of common warts (Verruca vulgaris) in adolescents and adults. The goal of the clinical trial is to compare outcome in healthy subjects 12 years of age and older with at least 3, but no more than 20, common warts (Verruca vulgaris) following treatment with Candin or placebo.
During the trial, participants will receive an injection of either Nielsen’s purified candida antigen (Candin) or a placebo every two weeks until either clinical clearance of the treatment wart is achieved, or a total of 10 injections have been administered. The primary endpoint of the trial is complete resolution of the treatment wart without recurrence at week 12.
Results are expected by the end of 2025.
“Warts are a cutaneous manifestation of human papilloma virus (HPV) infection. Therefore, an immunological therapy directed at eliminating the viral infection has the potential to be an improvement to removal of the wart versus acid, surgery, cryotherapy, and other methods that can damage skin,” adds H. Stewart Nielsen, Jr., Ph.D., Vice Chairman and Founder of Nielsen.
Nielsen is planning regulatory submissions in the US following the successful conclusion of the trial and has partnered with Maruho Co., Ltd. for the regulatory submission and commercialization of Candin in Japan. Nielsen is currently evaluating options and engaging in partnership discussions for commercialization in the US and other geographies.