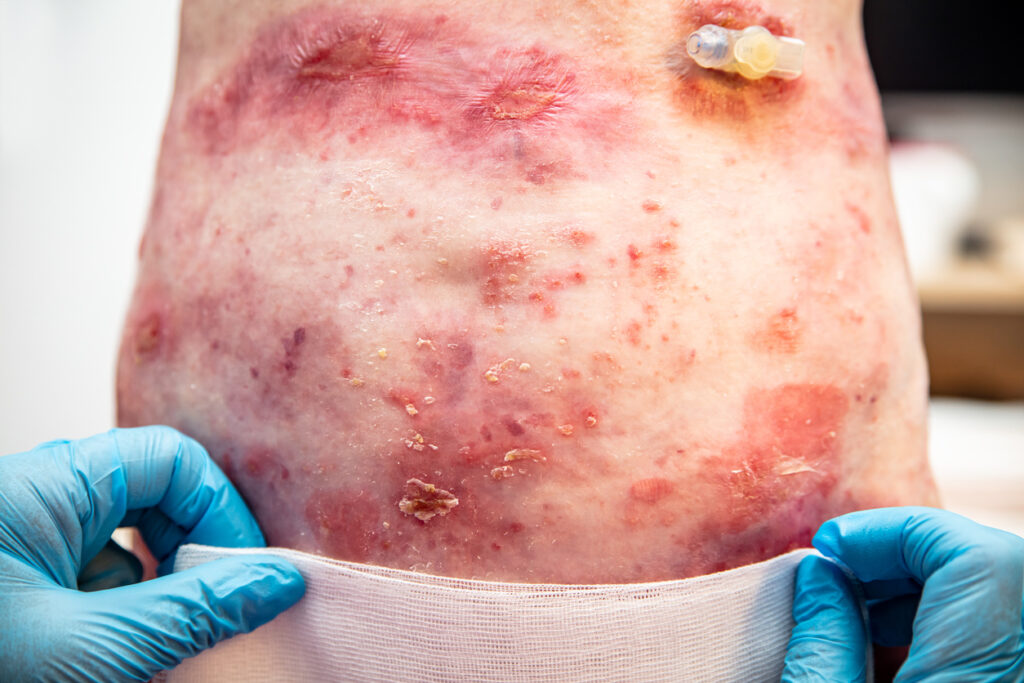
The European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) has issued a positive recommendation for the European Commission (EC) to approve Krystal Biotech, Inc.’s Vyjuvek (beremagene geperpavec-svdt, or B-VEC) for the treatment of wounds in patients with dystrophic epidermolysis bullosa (DEB) who have mutations in the collagen type VII alpha 1 chain (COL7A1) gene, starting from birth.
The CHMP’s positive opinion includes support for administration of B-VEC in either a health care setting (e.g., a clinic) or at home. If deemed appropriate by a healthcare professional, trained patients or caregivers may also apply B-VEC.
The final EC decision is anticipated in the second quarter of 2025. The decision will be applicable to all European Union member states, as well as Iceland, Norway, and Liechtenstein.If approved by the EC, the Company expects to market B-VEC under the registered European trademark, Vyjuvek.
“We are excited to be able to provide DEB patients with the first treatment that corrects the genetic defect and makes a true difference in their lives,” says Cristina Has, MD, Professor and Head of the Genodermatoses Clinic in the Department of Dermatology at the University of Freiburg in Germany, in a news release. “By addressing the very first stage in the complex pathophysiology of DEB, VYJUVEK is a landmark. It is amazing how simple and non-invasive its use is, even in infants.”
“We are very pleased that our patients, from birth, will have a simple, topical treatment that promotes durable wound closure, something that until now has been beyond the reach of any therapy,” adds Christine Bodemer, MD, PhD, Professor and Head of the Department of Dermatology at the Necker Enfants Malades Hospital in Paris. “This is a remarkable advance for DEB patients and a new approach to gene therapy for genodermatoses, revolutionary and remarkably innovative.”
The positive opinion issued by the CHMP is based on a comprehensive clinical dataset including results from the Company’s Phase 1/2 GEM-1 and Phase 3 GEM-3 studies, published in Nature Medicine and the New England Journal of Medicine, respectively, which collectively provided clear clinical evidence of successful COL7A1 gene delivery and durable wound closure following topical administration. The long-term safety and efficacy of B-VEC is further supported by results from the Company’s open label extension study completed in the United States as well as real-world experience with B-VEC since launching the United States in 2023.
“The CHMP’s recommendation for approval of VYJUVEK is an exciting step towards our goal of delivering the first ever corrective therapy to DEB patients across Europe,” says Suma Krishnan, President of Research and Development at Krystal Biotech. “The CHMP’s support for a broad label, including treatment of patients from birth and the option of patient or caregiver administration at home, are also fantastic outcomes for the DEB patients we aim to serve, broadening access and reducing barriers to starting on and staying on therapy.”
“Our team in Europe has been working tirelessly in preparation for Krystal’s first commercial launch outside of the United States and with the CHMP’s positive opinion we remain on track to launch in Germany around the middle of this year,” says Laurent Goux, Senior Vice President and General Manager of Europe at Krystal Biotech. “This will be the first of many launches in Europe, including a launch in France planned for later in 2025, as we advance diligently to ensure as many patients as possible benefit from access to VYJUVEK.”