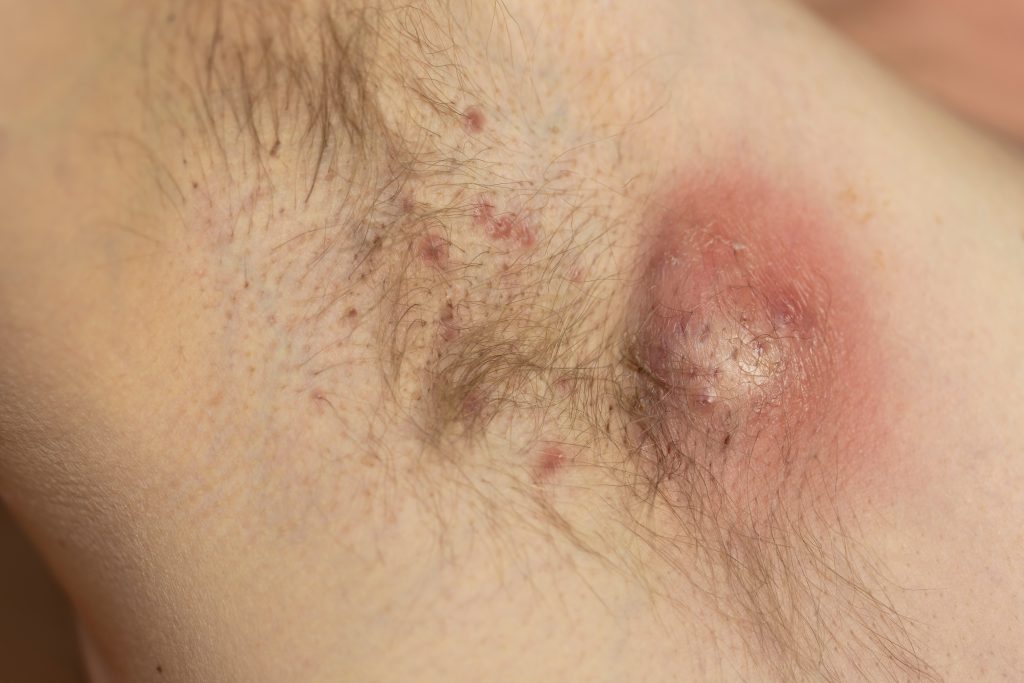
Bimekizumab (Bimzelx, UCB) improves clinical response, symptoms, severity, and quality of life in patients with hidradenitis suppurativa (HS) and results last up to two years, according to data from Phase 3 studies, BE HEARD I and BE HEARD II, and their open-label extension presented at the European Academy of Dermatology and Venereology (EADV) Congress in Amsterdam.
Bimekizumab is an interleukin (IL)-17A and IL-17F inhibitor, in adults with moderate-to-severe hidradenitis suppurativa (HS). In the U.S., bimekizumab is approved for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, adults with active psoriatic arthritis, adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation, and adults with active ankylosing spondylitis. Bimekizumab is not approved in the U.S. for the treatment of moderate-to-severe hidradenitis HS.
At Week 96, 85.4% (n=381/446) of patients treated with bimekizumab achieved HS Clinical Response 50 (HiSCR50). The more stringent endpoints, HiSCR75 and HiSCR100, were achieved by 77.1% (n=344/446) and 44.2% (n=197/446) of patients, respectively. Improvements in the severity of disease, reductions in draining tunnel count, and improvements in health-related quality of life were also maintained over two years, the study showed. Bimekizumab was generally well tolerated and no new safety signals were observed.
“The bimekizumab data presented at EADV 2024 showed maintained improvements in clinical response, symptoms, severity, and quality of life over two years. These findings are particularly encouraging given the need for new treatment options that offer sustained relief for patients,” says Professor Christos C. Zouboulis, President of the European Hidradenitis Suppurativa Foundation (EHSF) e.V., Director of the Departments of Dermatology, Venereology, Allergology and Immunology at Städtisches Klinikum Dessau, and Founding Professor of Dermatology and Venereology at the Brandenburg Medical School in Neuruppin, Germany.
A deeper dive into the studies
Patients who completed Week 48 in BE HEARD I and BE HEARD II could enroll in the open-label extension study and received bimekizumab every two weeks (Q2W) or every four weeks (Q4W) based on ≥90 percent HiSCR averaged from Weeks 36, 40, and 44. Results are shared for the bimekizumab total patient group.
HS Clinical Response:
– HiSCR50 was achieved by 79.9% (n=444/556) of bimekizumab patients at Week 48 and 85.4 percent (n=381/446) at Week 96.
– HiSCR75 was achieved by 64.0% (n=356/556) of bimekizumab patients at Week 48 and 77.1 percent (n=344/446) at Week 96.
– HiSCR90 was achieved by 42.3% (n=235/556) of bimekizumab patients at Week 48 and 57.6 percent (n=257/446) at Week 96.
– HiSCR100 was achieved by 30.2% (n=168/556) of bimekizumab patients at Week 48 and 44.2 percent (n=197/446) at Week 96.
– International HS Severity Score System (IHS4): Patients treated with bimekizumab saw a decrease in the severity of their HS symptoms as measured by the IHS4 score. The mean IHS4 score at baseline was 35.6±31.5 among bimekizumab patients. The percent change from baseline in IHS4 score at Week 48 among bimekizumab patients was maintained through Week 96 (-70.3±39.6 and -79.8±28.1 at Week 48 and Week 96, respectively).
– Draining tunnel (DT) count: The mean DTs at baseline was 3.8±4.3 among bimekizumab patients. The percent change from baseline in draining tunnel count at Week 48 among bimekizumab patients was maintained through Week 96 (–57.5 ±72.9 and –73.7±45.7%, at Week 48 and Week 96, respectively.
Health-related quality of life: The proportion of bimekizumab patients who achieved Dermatology Life Quality Index (DLQI) 0/1 at Week 48 was maintained through Week 96. The Mean DLQI Total at baseline was 11.0±6.8 among bimekizumab patients. Approximately one in three patients reported minimal or no impact of the disease on their health-related quality of life over two years (27.4% [n=151/155] and 33.9% [n=149/439] at Week 48 and Week 96, respectively).
Bimekizumab was generally well-tolerated over two years with no new safety signals observed. Over two years, 917/995 patients who received ≥1 dose of bimekizumab experienced a treatment-emergent adverse event (TEAE). Serious TEAEs were reported in 122 patients. Over two years, the most common TEAEs were hidradenitis, coronavirus infection, and oral candidiasis.
“In moderate to severe hidradenitis suppurativa, healthcare professionals and patients value long-term data when they are making treatment decisions. We are proud to present, for the first time, the bimekizumab two-year results at EADV 2024,” adds Fiona du Monceau, Executive Vice President, Head of Patient Evidence, UCB. “These longer-term data build on the 48-week results, demonstrating maintained response over two years, which is highly relevant for the hidradenitis suppurativa community.”