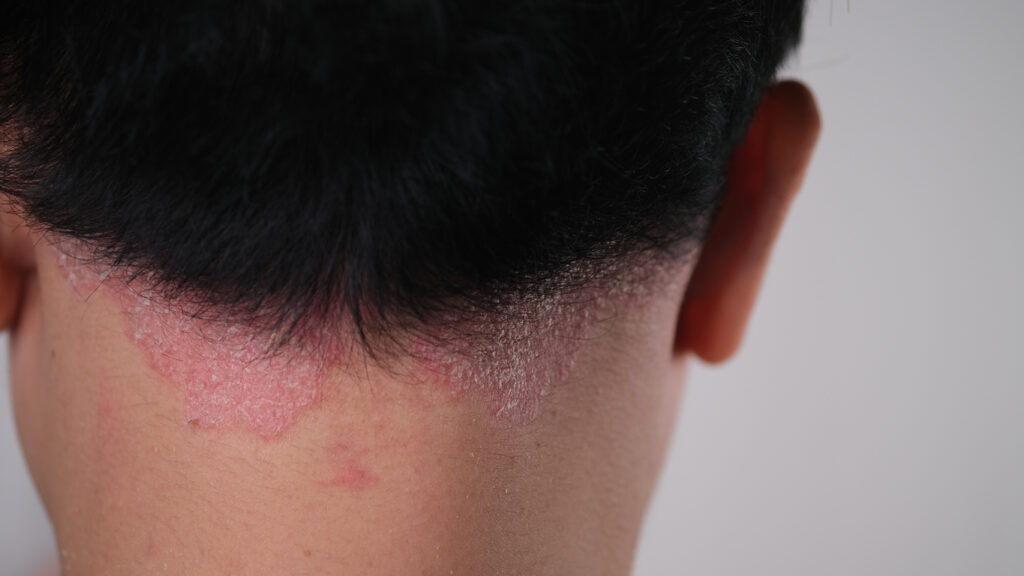
More than three times as many patients with scalp psoriasis taking deucravacitinib (Sotyktu, BMS) were clear or almost clear by Week 16 when compared to their counterparts in the placebo arm, according to data from the Phase 3b/4 PSORIATYK SCALP trial.
Specifically, 48.5% people taking the oral tyrosine kinase 2 (TYK2) inhibitor showed a statistically significant improvement in the scalp-specific Physician’s Global Assessment (ss-PGA) response of 0 or 1 (clear/almost clear) at 16 weeks. By contrast, 13.7% of people in placebo hit this mark.
The trial also met key secondary endpoints at Week 16, with a significantly higher percentage of patients achieving at least a 90% improvement in Psoriasis Scalp Severity Index (PSSI) response and a change from baseline (CFB) in scalp-specific itch with deucravacitinib treatment compared with placebo (PSSI 90: 38.8% versus 2.0%, respectively; mean CFB in scalp-specific itch -3.2 versus -0.7, respectively). In patients with Static Physician’s Global Assessment (sPGA) ≥3, a greater proportion achieved sPGA 0/1 with deucravacitinib treatment versus placebo (51.0% versus 4.3%).
Greater improvements were also reported by patients receiving deucravacitinib versus placebo, respectively, in achieving the minimum clinically important difference (MCID) for scalp-specific itch (41.7% versus 9.8%), pain (26.2% versus 11.8%) and flaking (53.4% versus 19.6%), as well as whole-body itch (39.8% versus 13.7%) numeric rating scale (NRS) scores.
The safety profile of deucravacitinib in PSORIATYK SCALP was consistent with findings in previously conducted clinical trials of deucravacitinib in psoriasis. The most common adverse events associated with deucravacitinib treatment in the PSORIATYK SCALP trial were nasopharyngitis, upper respiratory tract infection, acne, headache, COVID-19, and pustular acne (5.8%).
“These new results reinforce that oral Sotyktu is a safe and effective once-daily treatment for people living with moderate-to-severe psoriasis, with involvement of high impact areas such as the scalp,” says Mark Lebwohl, MD, Dean of Clinical Therapeutics at the Kimberly and Eric J. Waldman Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York City and an investigator and paid consultant for Bristol Myers Squibb, in a news release.
Real-word data supports benefits of deucravacitinib in psoriasis
In related news, deucravacitinib use in a real-word setting backs up the results of clinical studies in psoriasis.
Deucravacitinib was effective after six months of continuous treatment in real-world registry patients, which is consistent with efficacy outcomes observed in the POETYK PSO clinical studies in patients living with moderate-to-severe plaque psoriasis.
The Registry of Psoriasis Health Outcomes: A Longitudinal Real-World Collaboration Study (RePhlect) evaluated 118 patients, 108 of whom had moderate-to-severe plaque psoriasis. In an interim analysis, psoriasis patients in the overall group achieved statistically significant mean decreases in measures of disease severity (67.9% achieved Psoriasis Area and Severity Index (PASI) scores ≤3; PASI mean baseline score 6.3, change from baseline -4.1), percentage of affected Body Surface Area (BSA) scores (64.1% of patients achieved a BSA ≤3%; BSA mean baseline score 9.3, change from baseline -5.8) and Investigator’s Global Assessment (IGA) scores (46.8% achieved IGA scores of 0/1; IGA mean baseline score 2.7, change from baseline -1.2) from baseline to follow-up.
Similar results were observed in the subset of patients with moderate-to-severe plaque psoriasis.
“These data further demonstrate the safety and efficacy of deucravacitinib for the treatment of psoriasis in high-impact areas, such as the scalp, and include the first analysis from our RePhlect registry providing evidence highlighting the real-world benefit of Sotyktu for the treatment of moderate-to-severe plaque psoriasis,” says Daniel Quirk, MD, MPH, MBA, Senior Vice President of Worldwide Immunology and Neuroscience Medical Affairs at Bristol Myers Squibb. “We believe Sotyktu has the potential to be the systemic therapy that healthcare providers turn to when treating adult patients with moderate-to-severe psoriasis, especially those with scalp involvement. Overall, these promising results reinforce once-daily Sotyktu as a potential oral standard of care as we continue to lead in TYK2 innovation.”
Both studies were presented at the European Academy of Dermatology and Venereology (EADV) Congress in Amsterdam.