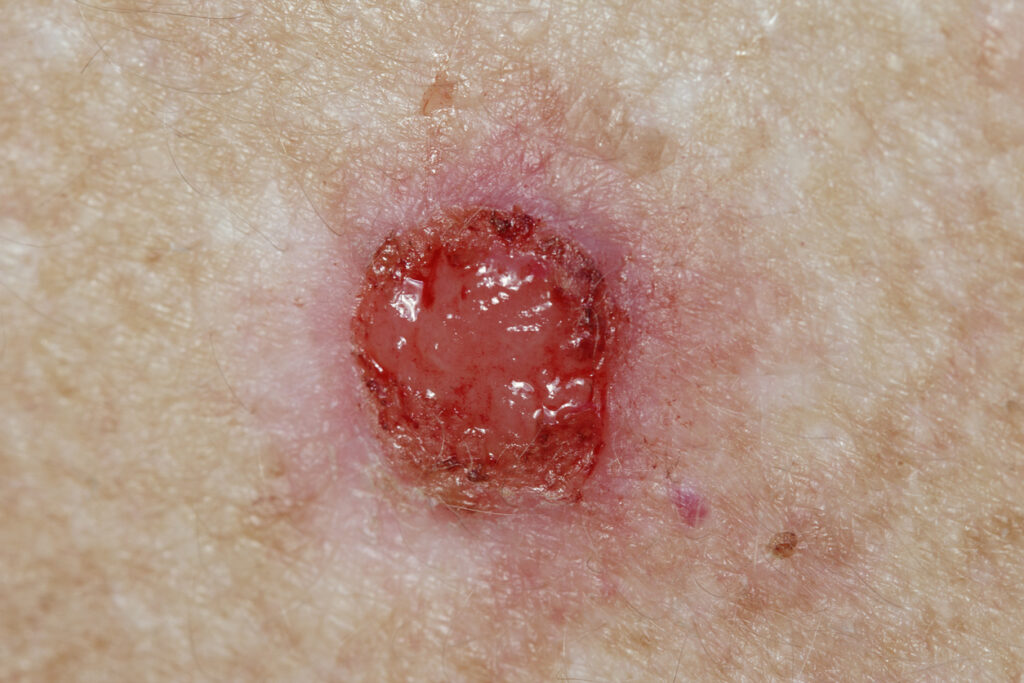
DermBiont’s SM-020 is performing well in an ongoing open label, multi-center Phase 2a study for the treatment of basal cell carcinoma (BCC), the Company reports.
SM-020 is a first-in-class, patient-applied, topical kinase inhibitor.
All nine treated BCCs (1 superficial, 4 nodular, and 4 infiltrating) across seven enrolled subjects to date showed “prompt, visible, and robust” tumor responses during treatment with SM-020 1% twice daily for 28 days, resulting in partial or total reduction of clinically visible tumor.
As a result of the new data, DermBiont has reprioritized SM-020 for the development of two lead orphan disease oncology indications: treatment of locally advanced basal cell carcinoma (laBCC) and prevention of BCC in Gorlin Syndrome patients.
DermBiont’s Phase 2a open-label trial is actively enrolling and treating up to 30 subjects with one to five superficial, nodular, or infiltrating primary BCCs. Lesions are being treated with SM-020 1% gel twice daily for 28 days, with the primary endpoint based on percent change from baseline in greatest tumor diameter at week 6. The safety of the drug product is being evaluated based on frequency of adverse events (AEs) and application site reactions (ASRs).
At the time of this release, seven subjects with a total of nine BCCs (1 superficial, 4 nodular, and 4 infiltrating) have been treated and show a prompt (within days of starting treatment) and robust clinical response to the application of SM-020 1% gel, clearly demonstrating the disease modifying potential of SM-020. All nine tumors have also demonstrated a partial or total reduction in clinically visible tumor.
“We are excited about these early results in BCC and the potential for a topical product for the treatment of laBCC and the prevention of BCCs in Gorlin Syndrome,” says Karl Beutner, MD, Ph.D., and CEO of DermBiont, in a news release. “An effective topical treatment would be welcomed by patients with laBCC given the limitations and side effects of the currently approved oral hedgehog inhibitors, while also providing patients with Gorlin Syndrome with an alternative to expensive and painful surgical procedures to remove chronic BCCs that result in significant scarring, require post-operative wound care, and lead to significant down time.”
Emma Taylor, MD, Chief Medical Officer at DermBiont, adds, “SM-020 in vitro is highly potent at killing BCC cells and has a well-defined mechanism of action that hits established tumorigenesis pathways known to be the cause of BCCs, so the robust response that we are seeing in the clinic is not surprising.”
SM-020 has been well tolerated to date with no drug-related adverse events and only rare and transient primarily mild-to-moderate severity application site reactions. The same drug product has also been shown to be extremely well tolerated by subjects with seborrheic keratoses (SK), having been administered by more than 100 subjects with SKs twice daily for at least 28 days in prior clinical trials.
In parallel to ongoing pivotal trial-enabling nonclinical and CMC scale-up work, DermBiont plans to seek Orphan Drug and Fast Track Designations for the treatment of laBCC and the prevention of BCC in patients with Gorlin Syndrome, to be followed by pivotal trials in two orphan indications for the approval of SM-020 for treatment of laBCC and prevention of BCC in Gorlin Syndrome patients. Following the reprioritization of SM-020 for laBCC and Gorlin Syndrome, Dermbiont is evaluating options for its SK program.