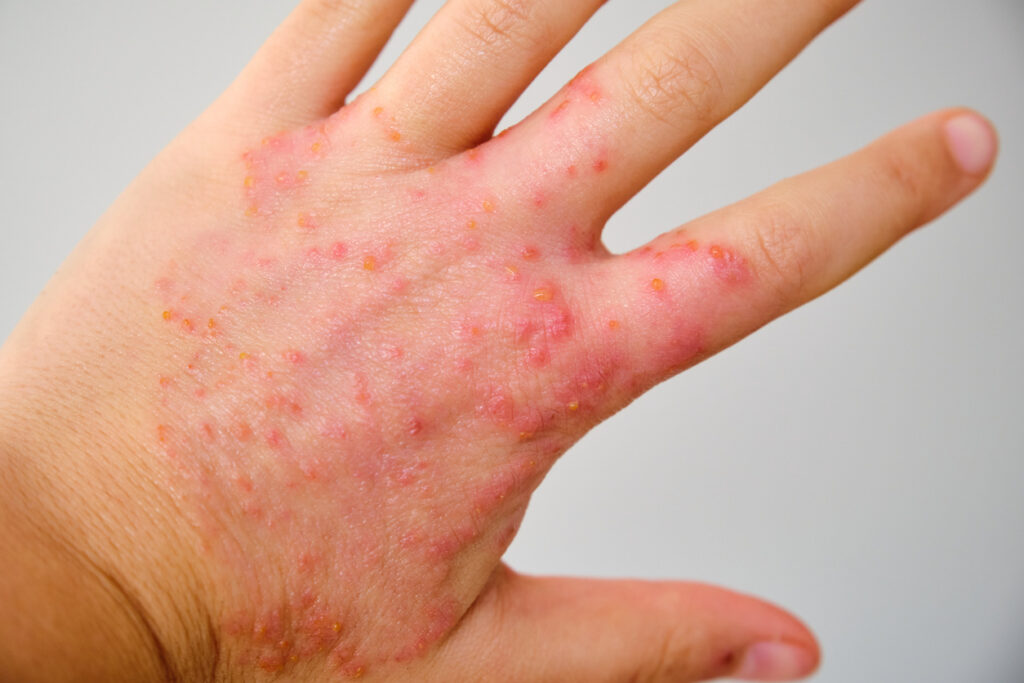
Delgocitinib cream met the primary and secondary endpoints in the DELTA 1 and DELTA 2 Phase 3 severe chronic hand eczema (CHE) trials.
Leo’s delgocitinib cream, a topical pan-Janus kinase (JAK) inhibitor, is under investigation and has not been approved by any health authority.
The primary objective for the randomized, double-blind, vehicle-controlled, multi-center phase 3 clinical trials (DELTA 1 and DELTA 2) was to evaluate the safety and efficacy of twice-daily applications of delgocitinib cream compared with cream vehicle in the treatment of adults with moderate to severe CHE who had inadequate response to, or for whom topical corticosteroids were medically inadvisable.
The new research is published in The Lancet.
The trials’ primary endpoint was the Investigator’s Global Assessment for chronic hand eczema treatment success (IGA-CHE TS) at Week 16. Treatment success was defined as an IGA-CHE score of 0 (clear) or 1 (almost clear), with at least a two-step improvement from baseline. Additional IGA-CHE scores included 2 (mild), 3 (moderate), and 4 (severe).
At week 16, a greater proportion of delgocitinib-treated patients versus cream vehicle patients had IGA-CHE treatment success in DELTA 1 and DELTA 2, the studies showed. The proportion of patients who reported adverse events was similar with delgocitinib DELTA 1, DELTA 2 and the cream vehicle arms. The most frequent adverse events occurring in at least 2% of patients were similar in both treatment groups, including COVID-19 and nasopharyngitis.
ICYMI: Reviewing the Data on Delgocitinib Cream for Chronic Hand Eczema
Key secondary endpoints at Week 16 included reduction of itch and pain scores of ≥4 points measured by the Hand Eczema Symptom Diary (HESD) from baseline to Week 16, as well as at least 75% improvement from baseline and at least 90% improvement from baseline on the Hand Eczema Severity Index (HECSI) at Week 16. The number of treatment-emergent adverse events from baseline to Week 16 defined the key safety endpoint of the trials.
“Historically, CHE has been under-researched, so the publication of the DELTA 1 and 2 trials in The Lancet is a positive step towards highlighting the condition, and in turn indicates the increased quality and rate of research in the space,” says Robert Bissonnette, MD, Chief Executive Officer and Medical Director at Innovaderm Research in Montreal, Quebec, Canada, in a news release. “Publication of milestones like this have a valuable role in our efforts to improve the day-to-day reality of those living with CHE.”
Subjects who completed 16 weeks of treatment with delgocitinib cream or cream vehicle twice daily in trials DELTA 1 or DELTA 2 were offered to roll over to the DELTA 3 open-label, multi-site 36-week extension trial. The purpose of this extension trial was to evaluate the long-term safety of delgocitinib.