May is Ichthyosis Awareness Month, and TDD has curated its content on congenital ichthyosis (CI) to help raise awareness of the condition and shine a light on promising new therapies in the pipeline.
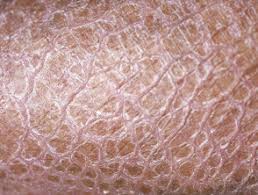
Ichthyosis is characterized by dry, scaling skin that may be thickened or very thin, according to the Foundation for Ichthyosis & Related Skin Types. Each year, more than 16,000 babies are born with some form of ichthyosis.
How to Think Like an Ichthyosis Expert
Christopher Bunick, MD, PhD, an associate professor of dermatology at Yale University in New Haven, CT, shares some congenital ichthyosis (CI) cases and discusses the importance of understanding patient and family expectations about CI treatment.
Congenital Ichthyosis Treatment Update
Christopher Bunick, MD, PhD, an associate professor of dermatology at Yale University in New Haven, CT, discusses findings from the phase 3 maximal use arm of the ASCEND Trial that looked at topical isotretinoin 0.05% (TMB-001) in congenital ichthyosis. The upshot? Topical isotretinoin, even when applied to 90% of a patient’s body surface area, showed minimal systemic absorption.
TDD Talks to Brian Hilberdink, LEO Pharma’s EVP of North America, About Congenital Ichthyosis
LEO Pharma recently acquired TMB-001 and other assets from Timber Pharmaceuticals following Timber’s Chapter 11 bankruptcy filing.
TMB-001 is an investigational topical reformulation of isotretinoin that is being studied in congenital ichthyosis (CI). The reformulation has received orphan, fast track, and breakthrough designation by the FDA. TMB-001 has shown positive results in Phase 2, with Phase 3 recruitment currently ongoing in the US.
Brian Hilberdink, LEO Pharma’s EVP of North America, chatted with TDD about the acquisition and how it fits in with LEO Pharma’s larger plans
Read the full interview here.
Photo Credit: DermNet