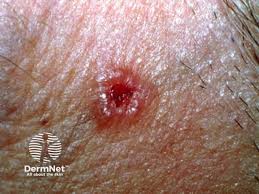
Medicus Pharma Ltd.’s phase 2 clinical study (SKNJCT-003) has now randomized more than 50% of the 60 patients expected to be enrolled in the study of D-MNA in nodular basal cell carcinoma.
D-MNA is noninvasive treatment consisting of a micro-array of needles containing doxorubicin. The study is currently underway in nine clinical sites in United States.
The Company also announced that it is on track to complete an interim data analysis before the end of Q1 2025 and to submit its findings to the United States Food and Drug Administration (FDA) as part of a package seeking a Type C meeting with the FDA in Q2 2025.
The purpose of the Type C meeting is to formally discuss the product development and gain further alignment on the clinical pathway. The Company’s aim is to gain FDA’s consent to fast-track the clinical development program.
SKNJCT-003, is designed to be a randomized, double-blind, placebo-controlled (P-MNA), multi-center study enrolling up to 60 subjects presenting with BCC of the skin. The study will evaluate the efficacy of two dose levels of D-MNA compared to a placebo control. The participants will be randomized 1:1:1 to one of three groups: a placebo-controlled group receiving P-MNA, a low-dose group receiving 100g of D-MNA, and a high-dose group receiving 200g of D-MNA.
The high-dose, 200g D-MNA, proposed in the study is the maximum dose that was used in the Company’s Phase 1 safety and tolerability study (SKNJCT-001) completed in March 2021.
SKNJCT-001 met its primary objective of safety and tolerability.
The investigational product, D-MNA was well-tolerated across all dose levels in all thirteen (13) participants enrolled in the study, with no dose-limiting toxicities (DLTs), or serious adverse events (SAEs). Furthermore, there were no systemic effects or clinically significant abnormal findings in laboratory parameters, vital signs, ECGs, and physical examinations. The study also describes the efficacy of the investigational product, D-MNA, with 6 participants experiencing complete responses. The complete response is defined as the disappearance of BCC histologically in the final excision at the end of study visit. The participants profile demonstrating complete responses was diverse, and all participants (6/6) had nodular subtype of BCC.
PHOTO CREDIT: DermNet