The Search for Steroid-sparing Options in PsO: PEPITEM and Its Tripeptide Sequence Shows Promise
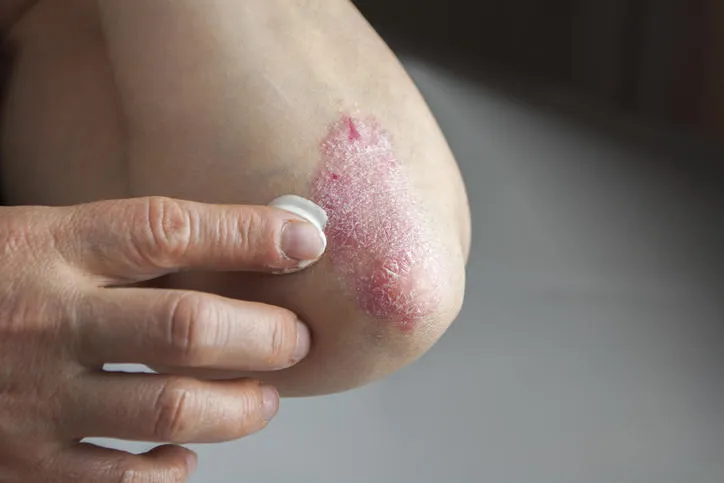
PEPITEM, a natural molecule, and the tripeptide sequence derived from it may reduce the severity of psoriasis when applied topically in an emollient cream.
