Icotrokinra Shows Efficacy and Safety in Adolescents With Moderate to Severe PsO
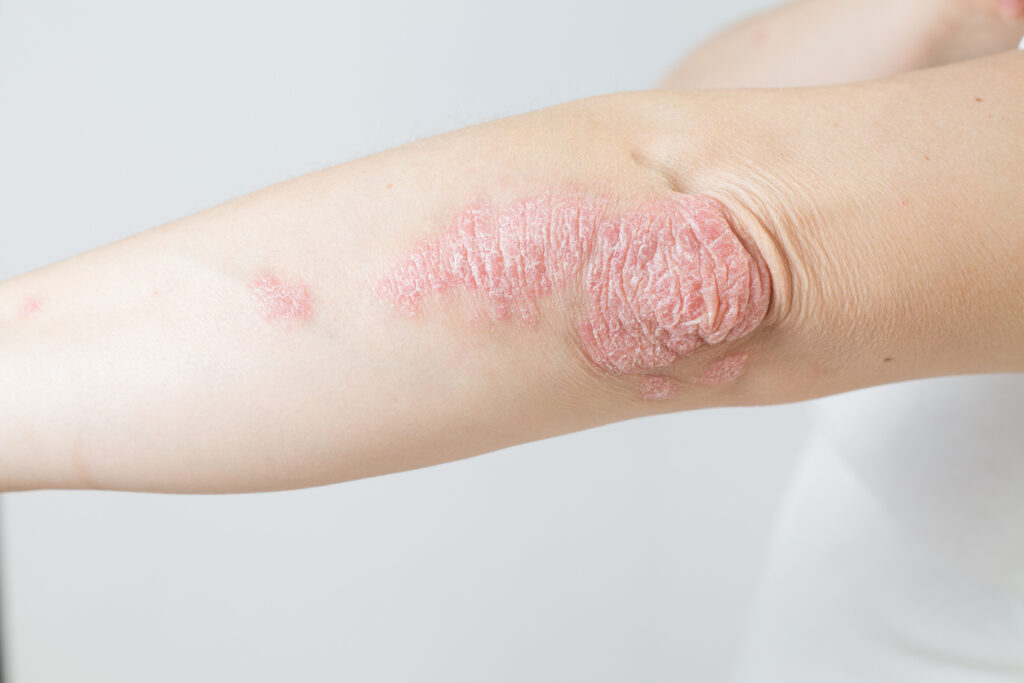
Three-quarters of adolescents with plaque psoriasis (PsO) treated with investigational icotrokinra (JNJ-2113) achieved completely clear skin with no new safety signals identified, according to data from a subgroup analysis of ICONIC-LEAD.
