Milestone Alert: Soligenix Enrolls Enough Patients for Planned Interim Analysis of HyBryte in CTCL
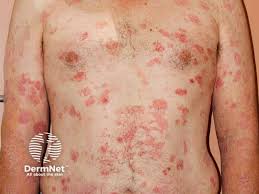
Soligenix, Inc. has completed the planned enrollment of 50 patients necessary for the interim analysis in its 80 patient confirmatory Phase 3 double-blind, placebo-controlled study evaluating synthetic hypericin (HyBryte) in the treatment of cutaneous T-cell lymphoma (CTCL).
