2025 SID Annual Meeting Coverage: Mayo Clinic Insights on Biologics and Patient Outcomes in HS
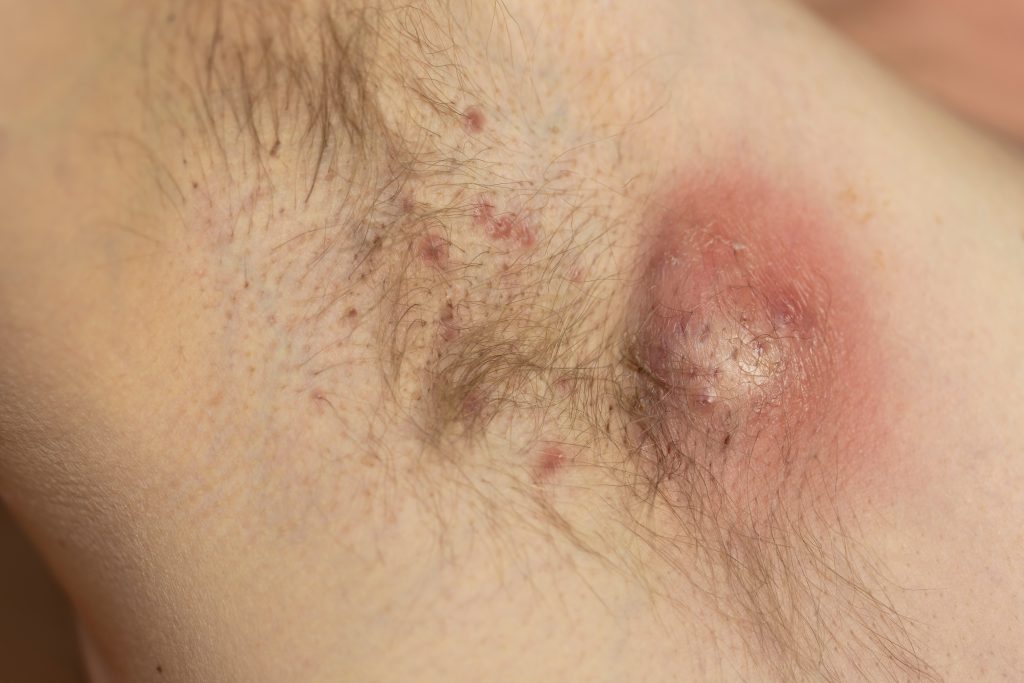
Patients with hidradenitis suppurativa (HS) who were treated with biologics were significantly less likely to be uninsured, covered by Medicaid, or covered by other government programs, compared with those who did not receive biologics, according to new research presented at the 2025 annual meeting of the Society for Investigative Dermatology in San Diego, CA.
