Hormone-disrupting Chemicals From Plastics and Ultra-processed Food Linked to HS
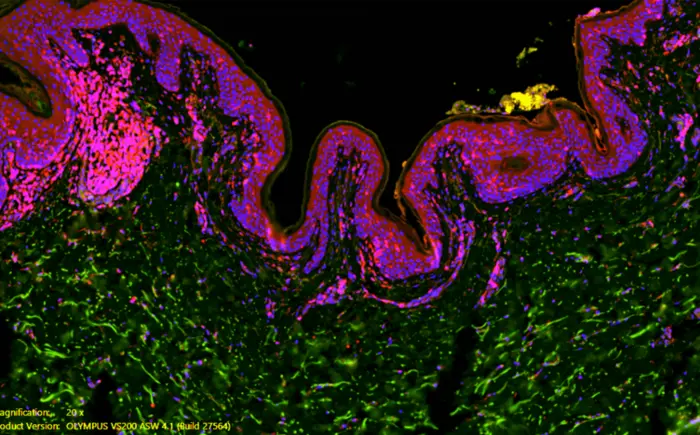
Hormone-disrupting chemicals commonly found in ultra-processed food and single-use water bottles may contribute to the development of or worsen hidradenitis suppurativa (HS) in some people, according to a small study out of Johns Hopkins Medicine.
