Study: Just One-third of Early Lyme Patients With Ongoing Symptoms Seek Care
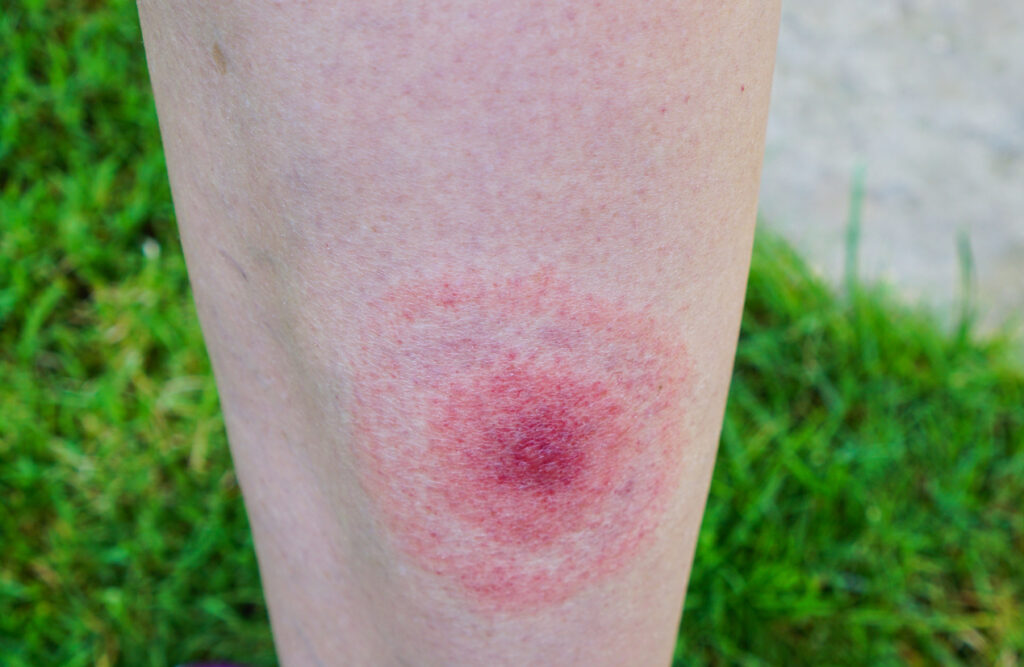
Many patients with Lyme disease who experience early and ongoing symptoms are not seeking medical care, a new study suggests.

Many patients with Lyme disease who experience early and ongoing symptoms are not seeking medical care, a new study suggests.