AD Pipeline Watch: Aclaris Therapeutics Initiates Phase 2 Trial of Bosakitug
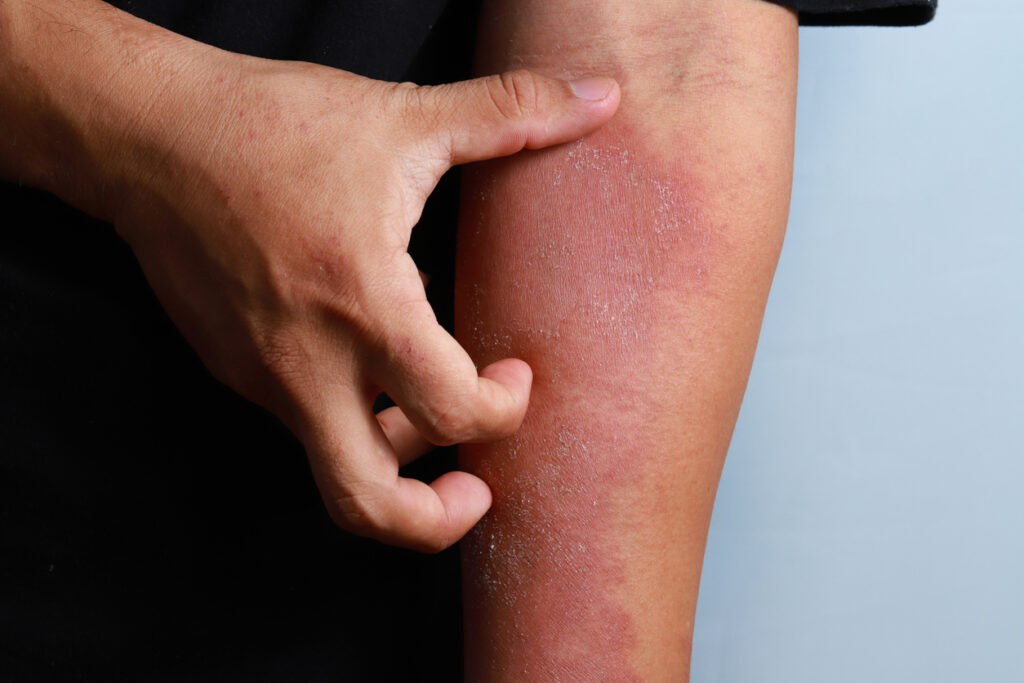
Aclaris Therapeutics, Inc. has initiated a randomized, double-blind, placebo-controlled Phase 2 trial of bosakitug (ATI-045) in patients with moderate-to-severe atopic dermatitis (AD).
