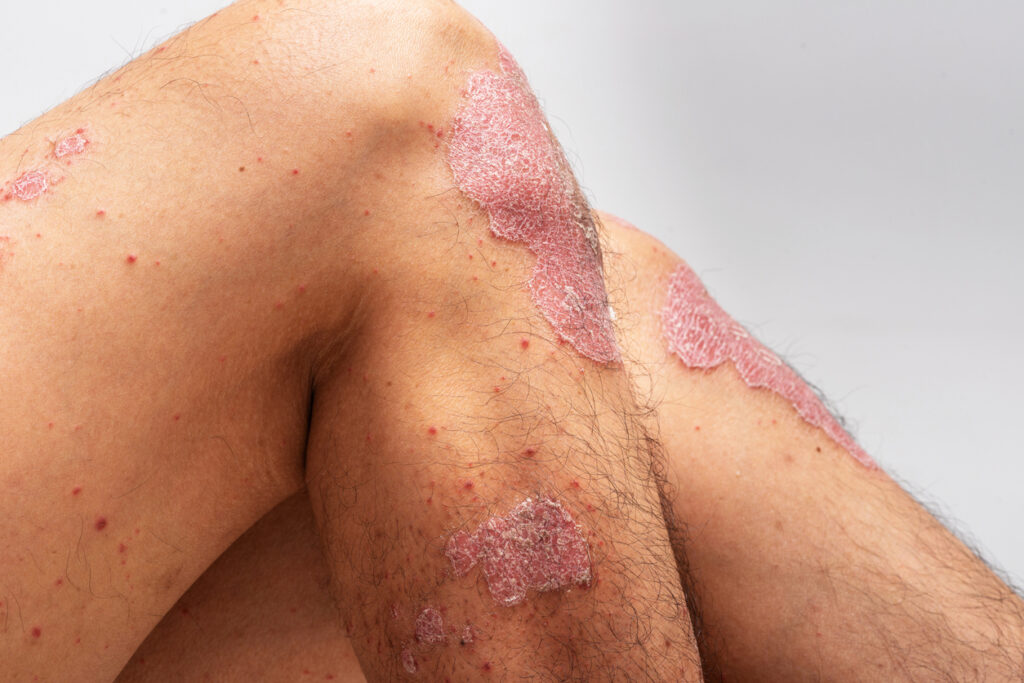
Can-Fite BioPharma Ltd. has started a Phase 3 psoriasis study of its oral drug Piclidenoson with the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Piclidenoson is a novel, oral first-in-class, A3 adenosine receptor agonist (A3AR) small molecule. The drug’s mechanism of action entails inhibition of interleukin 17 and 23 (IL-17 and IL-23) and the induction of apoptosis of patients’ skin cell keratinocytes involved with the disease pathogenicity.
Patient enrollment will be initiated in Europe, and US and Canada are expected to follow.
Aimed at Psoriasis Patients
The randomized, double-blind, placebo-controlled Phase 3 study is aimed at demonstrating clinical safety and efficacy for the treatment of patients with moderate-to-severe plaque psoriasis. Patients will be treated with 3mg twice daily orally Piclidenoson tablets or placebo. The co-primary efficacy objectives of this study are the proportion of subjects who achieve a Psoriasis Area and Severity Index (PASI) score response of ≥75% (PASI 75) and the proportion of subjects who achieve a Static Physician’s Global Assessment (sPGA) at of 0 or 1 at Week 16. The FDA requested two Phase 3 safety and efficacy studies. It also encouraged the Company to enroll adolescent patients due to the strong safety profile of the drug demonstrated over the development history and prior clinical studies.
“We are excited to initiate the Phase 3 study. We believe that Piclidenoson’s oral dosage and excellent safety record, together with its progressive effectiveness over time, make it an ideal drug for the chronic treatment of psoriasis,” says Can-Fite CEO Motti Farbstein, in a news release.
Upon positive conclusion of the Phase 3 study, the Company plans to submit a New Drug Application (NDA) to the U.S. FDA and Marketing Authorization Plan (MAA) to the EMA.