First approved long-acting injectable for HIV
by Bob Kronemyer
The first injectable and complete regimen for HIV-infected adults was approved in January by the Food and Drug Administration (FDA).
Cabenuva (ViiV Healthcare and Janssen Pharmaceutica) is a combination of cabotegravir, an HIV integrase strand transfer inhibitor (INSTI), and rilpivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), which is administered intramuscularly once a month.
Candidates for the extended-release injectable suspensions of the 2 drugs for concurrent administration are virologically suppressed (HIV-1 RNA less than 50 copies/mL) adults who are on a stable antiretroviral regimen, along with no history of treatment failure and with no known or suspected desistance to either constituent drug.
Cabenuva greatly simplifies an HIV treatment regimen while facilitating the healthcare provider’s monitoring adherence to an antiretroviral treatment regimen by injecting the patient in the office.
FDA approval of Cabenuva, and previous approval in Canada in March 2020, were based on 2 randomized but open-label parallel-group phase 3 trials comparing virologic suppression rates between Cabenuva and a standard oral 3-drug regimen over a period of 48 weeks: ATLAS (Antiretroviral Therapy as Long-Acting Suppression) in patients previously treated with oral antiretrovirals and FLAIR (First Long-Acting Injectable Regimen) in antiretroviral naïve patients.1
The ATLAS study
For the ATLAS study, 616 HIV patients were equally randomized to either continue their current oral antiretroviral regimen or switch to cabotegravir plus rilpivirine. The latter group initially took a 30 mg oral dose of cabotegravir and a 25 mg oral dose of rilpivirine for approximately 4 weeks; then, depending on tolerability and safety, patients began long-acting injectable therapy, which comprised loading doses of cabotegravir 600 mg and rilpivirine 900 mg, followed by 400 mg and 600 mg, respectively, once every 4 weeks (n = 308).
At trial entry, patients had been receiving their current antiretroviral regimen for a median of 4.3 years, mostly a 2 NRTI backbone, plus a NNRTI (50%), an INSTI (33%) or a protease inhibitor (PI) (17%).
At week 48, for the primary endpoint, only 1.6% of patients in the injection group and only 1.0% of patients in the oral therapy group had HIV-1 RNA levels ≥ 50 copies/mL, for an adjusted difference of 0.6% (95% confidence interval [CI]: 1.2 to 2.5).
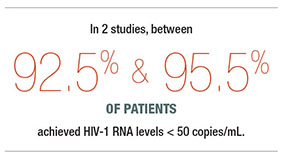
Conversely, 92.5% and 95.5% of patients in the injection and oral therapy groups, respectively, achieved HIV-1 RNA levels < 50 copies/mL, for an adjusted difference of 3% (95% CI: 6.7 to 0.7).
The 10-item HIV Treatment Satisfaction Questionnaire (HIVTSQ) total score revealed an adjusted mean of 5.68 points higher from baseline in the injection group than the oral therapy group.
Among patients in the injection group, 97% of respondents (266 of 273) to a single-item question about preference for long-acting vs. oral therapy at week 48 selected the long-acting injectable regimen.
However, 3 patients in the injection group had confirmed virological failure: 2 with HIV-1 subtype A/A1 and one with subtype AG.
HIV-1 RNA samples taken at the time of virologic failure detected rilpivirine resistance-associated reverse-transcriptase mutations in all three cases: E138A, E138K plus V108I and E138E/K plus the integrase mutation N155H in one patient each.
The FLAIR study
For the FLAIR study, 629 HIV patients received induction therapy with an oral fixed dose combination of dolutegravir 50 mg, abacavir 600 mg, and lamivudine 300 mg, once daily for 20 weeks.
In total, 566 patients who attained plasma HIV-1 RNA levels < 50 copies/mL after 16 weeks continued enrollment in the study, while 63 patients withdrew before randomization, mostly due to a lack of efficacy.
The remaining 566 patients were equally randomized to continue oral dolutegravir/abacavir/lamivudine or switch to cabotegravir plus rilpivirine, with the same post-induction regimen/methodology used in the ATLAS study. But only 278 of the 283 patients randomized to the injection group started, because the other 5 patients withdrew after the 4 weeks of oral therapy.
At week 48, only 2.1% of patients in the injection group and only 2.5% of patients in the dolutegravir/abacavir/lamivudine group had an HIV-1 RNA level of ≥ 50 copies/mL, for an adjusted difference of 0.4% (95% CI: 2.8 to 2.1), again the primary endpoint.
In contrast, 93.6% and 93.3% of patients, respectively, achieved HIV-1 RNA levels < 50 copies/mL, for an adjusted difference of 0.4% (95% CI: 3.7 to 4.5).
The total HIVTSQ score for satisfaction with current treatment compared to induction treatment at week 48 was 4.1 points higher in the injection group than in the oral therapy group.
In addition, 99% of patients in the injection group who responded to the single-item question about preference for long-acting or oral therapy at week 48 (257 of 259 patients) selected the long-acting injectable regimen.
But 4 patients in the injection group had confirmed virological failure: one had suspended oral lead-in treatment and 3 developed NNRTI and INSTI resistance mutations.
Also, at week 96, 3.2% patients in the injection group and 3.2% patients in the oral therapy group had an HIV-1 RNA level of ≥50 copies/ mL, for an adjusted difference of 0.0% (95% CI: 2.9 to 2.9).
Conversely, at the same time point, 86.6% and 89.4% of patients, respectively, achieved HIV-1 RNA levels <50 copies/mL, for an adjusted difference of 2.8% (95% CI: 8.2 to 2.5).
Furthermore, at week 96, a significantly higher level of treatment satisfaction, based on total HIVTSQ scores, was reported by the injection group than the oral therapy group, for a between-group difference in adjusted mean change from baseline of 2.3 points (P < 0.001).
No new patients treated with injection had confirmed virological failure between weeks 48 and 96; however, one oral therapy recipient developed confirmed virological failure at week 64, with no treatment-emergent resistance.
For the 2 studies combined, Cabenuva was generally very well tolerated, with short-lived injection site reactions (96% mild to moderate), fever (8%) and myalgia (3%). Fatigue, headache, nausea, and dizziness were rare. There were also no reports of hepatotoxicity in either study.
Overall, 4% and 2% of the injection and control groups, respectively, discontinued treatment because of adverse events in the 2 studies, mostly because of injection site reactions, hepatitis A, acute hepatitis B, headache and diarrhea.
If a patient plans to miss a scheduled injection visit by more than 7 days, daily oral therapy should replace up to 2 consecutive monthly injection visits. The recommended oral daily dose is one 30-mg tablet of cabotegravir and one 25-mg tablet of rilpivirine.
REFERENCE
1. Markham A. Cabotegravir plus rilpivirine: first approval. Drugs (2020) 80:915–922. doi:org/10.1007/s40265-020-01326-8