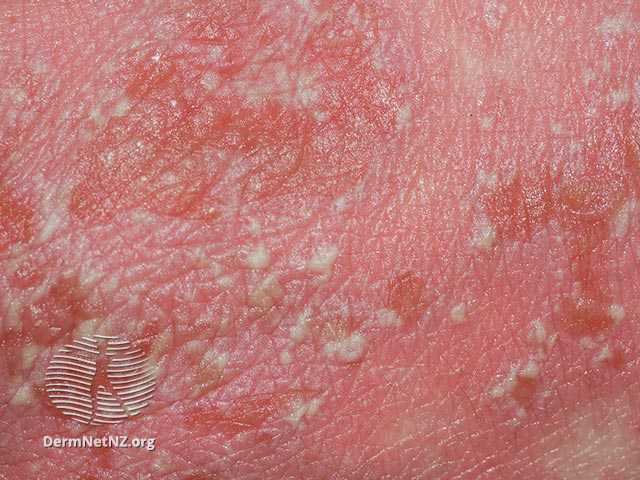
Boehringer Ingelheim and LEO Pharma are teaming up to commercialize and advance the development of spesolimab (Spevigo), an interleukin-36 (IL-36) blocker approved to treat generalized pustular psoriasis (GPP).
This new partnership extends beyond GPP, with an opportunity to investigate the potential of spesolimab in additional skin conditions with high unmet medical need in which IL-36 is implicated.
Under the terms of the agreement, LEO Pharma will be responsible for commercialization and further development of spesolimab. The transaction is anticipated to close in the second half of 2025, subject to merger control clearance, with Boehringer Ingelheim set to receive EUR 90 million as upfront payment, along with milestone payments and tiered royalties.