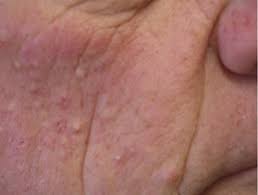
ATR-04 may help address epidermal growth factor receptor inhibitor (EGFRi)-induced dermal toxicity, which affects approximately 150,000 people in the US, according to preclinical data presented on at the Society of Investigative Dermatology (SID) 2024 Annual Meeting in Dallas, TX.
ATR-04 is a live biotherapeutic product candidate consisting of an S. epidermidis strain that was isolated from a healthy volunteer and engineered to be safer by deleting an antibiotic resistance gene and engineering auxotrophy to control the growth of ATR-04.
ATR-04 is in development for EGFRi-associated skin toxicity, which is caused by the suppression of skin immunity by EGFRis and subsequent inflammation and often elevated levels of interleukin(IL)-36γ and S. aureus..
ATR-04 led to a decrease in S. aureus across multiple models, the studies showed. In in vitro skin models, ATR-04 treatment led to a 96% reduction in methicillin-resistant S. aureus (MRSA) compared to untreated skin. In ex vivo pig skin treated with ATR-04, there was a ~99% reduction in MRSA compared to untreated skin. Additionally, in human skin models treated with erlotinib, an EGFRi, ATR-04 reduced IL-36γ by 75% to levels comparable to untreated skin models. Finally, ATR-04 increased human beta defensin, a key protein involved in host defenses, by 18-fold compared to vehicle. Together, the data show that ATR-04 can address multiple drivers of EGFRi-induced skin toxicity.
“These data show robust preclinical activity of ATR-04 in models of EGFRi-induced dermal toxicities that support an upcoming Investigational New Drug (IND) application to the FDA for a Phase 1b in patients undergoing EGFR inhibitors with dermal toxicity,” says Travis Whitfill, Azitra’s co-founder and COO, in a news release. “We anticipate the expansion of our clinical-stage pipeline this year with ATR-04.”
PHOTO CREDIT: DermNet