J&J Asks FDA To Add PsA Joint Damage Inhibition Data to Guselkumab (Tremfya) Label
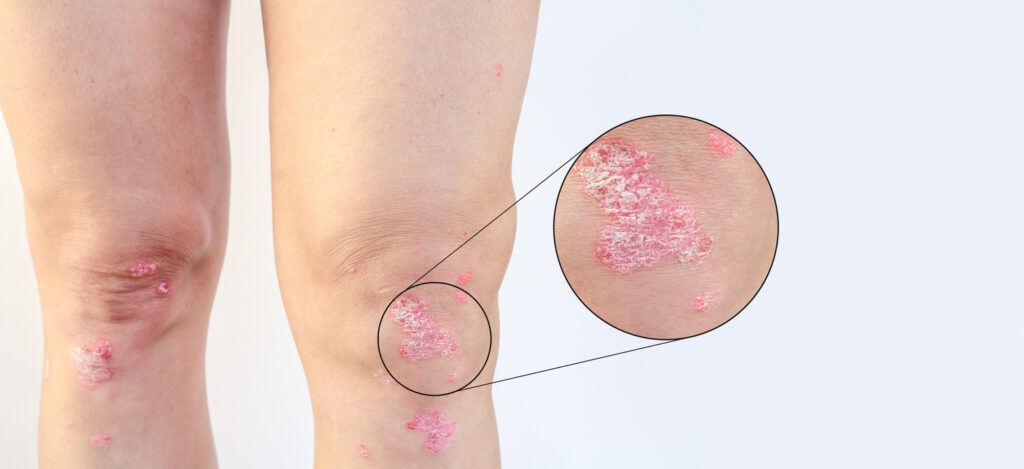
Johnson & Johnson submitted a supplemental Biologics License Application (sBLA) to the U.S. Food and Drug Administration (FDA) seeking approval to include new evidence on the guselkumab (Tremfya) label for the inhibition of progression of structural damage in adults with active psoriatic arthritis (PsA). The submission is supported by the Phase 3b APEX study in […]
